128-62-1
| Name | (-)-noscapine |
|---|---|
| Synonyms |
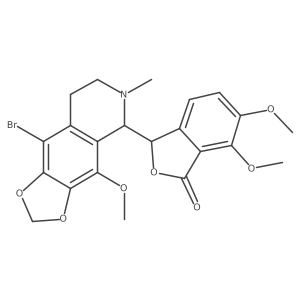
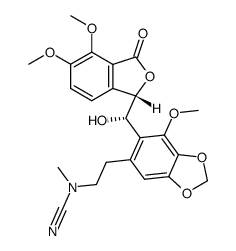
(3S)-3-[(5R)-6-methyl-4-(methyloxy)-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinolin-5-yl]-6,7-bis(methyloxy)-2-benzofuran-1(3H)-one
MFCD00069316 Coscopin Noscapal Vadebex Vadebe [3H]-Noscapine l-α-Narcotine Narcosine Narcotussin Key-tusscapine l-a-Narcotine Longatin Capval noscapine Coscopin (VAN) Gnoscopine Methoxyhydrastine Terbenol Narcotine (8CI) Nipaxon Tusscapine (S,R)-Noscapine (-)-α-Narcotine Narcotine Opianine Terial Eil Noscapalin (3S)-6,7-Dimethoxy-3-[(5R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinolin-5-yl]-2-benzofuran-1(3H)-one Coscotabs [S-(R*,S*)]-6,7-Dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)-1(3H)-isobenzofuranone l-a-2-Methyl-8-methoxy-6,7-methylenedioxy-1-(6,7-dimethoxy-3-phthalidyl)-1,2,3,4-tetrahydroisoquinoline Narcompren Opian (-)-noscapine Opianin Nectadon Nicolane EINECS 204-899-2 Lyobex (-)-Narcotine |
| Description | Noscapine is an orally administrable drug used worldwide for cough suppression, primarily mediated by its σ-receptor agonist activity, and possess anticancer activity.Target: σ-receptorin vitro: Noscapine is a phthalideisoquinoline alkaloid from opium, is a recently discovered anticancer drug and is currently under investigation in phase-I/II clinical trials for the treatment of leukemia and lymphoma. Noscapine causes few or no side effects and has been widely used as a cough suppressant in developing countries. Noscapine has been demonstrated to interact with microtubules. Interestingly, unlike many other microtubule-targeting agents such as Paclitaxel and Nocodazole, Noscapine does not obviously affect the total amount of microtubule polymers in cells; instead, it significantly increases the time microtubules spend in the pause state. The alteration of microtubule dynamics then activates the spindle checkpoint and arrests cell cycle progression at mitosis, leading to apoptotic cell death. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 565.3±50.0 °C at 760 mmHg |
| Melting Point | 174-176ºC |
| Molecular Formula | C22H23NO7 |
| Molecular Weight | 413.421 |
| Flash Point | 295.7±30.1 °C |
| Exact Mass | 413.147461 |
| PSA | 75.69000 |
| LogP | 2.83 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 24/25 |
| RIDADR | UN 1544 |
| WGK Germany | 3 |
| RTECS | RD2625000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~14% 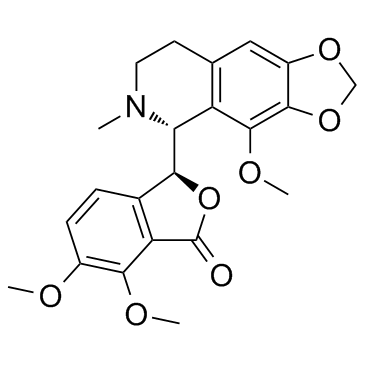
128-62-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 22, # 8 p. 2983 - 2987 |
|
~% 
128-62-1 |
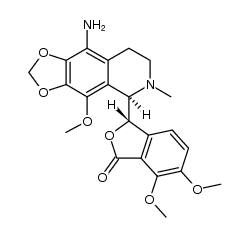
| Literature: Archiv der Pharmazie, , vol. 311, # 8 p. 664 - 671 |
|
~% 
128-62-1 |
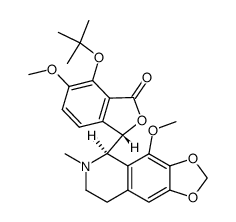
| Literature: Archiv der Pharmazie, , vol. 311, # 8 p. 664 - 671 |
|
~% 
128-62-1 |
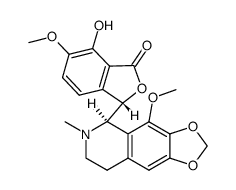
| Literature: Naunyn-Schmiedebergs Archiv fuer Experimentelle Pathologie und Pharmakologie, , vol. 184, p. 327,329 Journal of the Chemical Society, , p. 1087,1091 |
|
~% 
128-62-1 |
| Literature: Archiv der Pharmazie, , vol. 311, # 8 p. 664 - 671 |
|
~11% 
128-62-1 |
| Literature: Acta Chimica Academiae Scientiarum Hungaricae, , vol. 103, # 4 p. 343 - 353 |
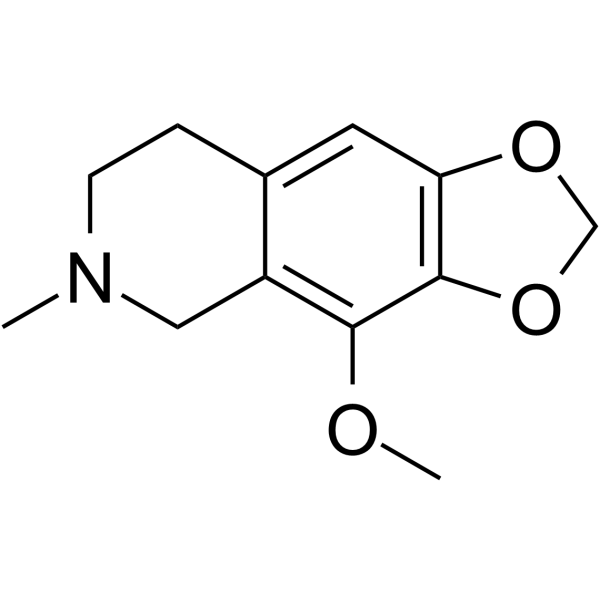
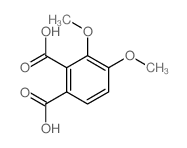
| Precursor 5 | |
|---|---|
| DownStream 8 | |