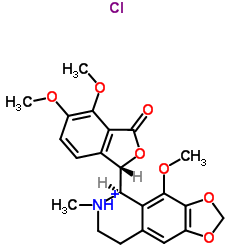
912-60-7
| Name | Noscapine hydrochloride,(3S)-6,7-Dimethoxy-3-[(5R)-5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl]-1(3H)-isobenzofuranonehydrochloride |
|---|---|
| Synonyms |
Narcosine hydrochloride
longatinhydrochloride EINECS 213-014-9 Terbenol hydrochloride Capval NOSCAPINE BASE / HCL MFCD00082546 nectadonhydrochloride Nectadon hydrochloride Opian hydrochloride Vadebex hydrochloride lyobexhydrochloride coscopinhydrochloride Coscopin hydrochloride 1,3-Dioxolo[4,5-g]isoquinolinium, 5-[(1S)-1,3-dihydro-4,5-dimethoxy-3-oxo-1-isobenzofuranyl]-5,6,7,8-tetrahydro-4-methoxy-6-methyl-, chloride, (5R)- (1:1) Narcompren hydrochloride opianhydrochloride capvalhydrochloride Opianine hydrochloride vadebexhydrochloride (5R)-5-[(1S)-4,5-Dimethoxy-3-oxo-1,3-dihydro-2-benzofuran-1-yl]-4-methoxy-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinolin-6-ium chloride Methoxyhydrastine hydrochloride Noscapine HCl Noscapine hydrochloride Narcotine hydrochloride |
| Description | Noscapine hydrochloride ((S,R)-Noscapine hydrochloride) is an orally active phthalideisoquinoline alkaloid with potent antitussive. Noscapine hydrochloride exerts its antitussive effects by activating sigma opioid receptors and is a non-competitive Bradykinin inhibitor. Noscapine hydrochloride disrupts microtubule dynamics, induces mitotic arrest and apoptosis. Noscapine hydrochloride possesses anticancer, neuroprotective, anti-inflammatory activities, and can crosse the blood-brain barrier[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
Sigma opioid receptors[4]Bradykinin[5]Apoptosis[1] |
| In Vitro | Noscapine (0-1000 μM; 0-96 hours; rat C6 glioma cells) treatment inhibits cell viability of rat C6 glioma in vitro in a dose- and time-dependent manner. Noscapine inhibits the viability of rat C6 glioma cells with an IC50 of 250 μM at 72 hours[1]. Noscapine exposure causes abnormal S-phase reentry, increases mitotic arrest and results in excessive DNA accumulation[1]. Cylindromatosis increases the ability of noscapine to induce mitotic arrest and apoptosis. Cylindromatosis enhances the effect of noscapine on microtubule polymerization and promotes noscapine binding to microtubules[2]. Cell Proliferation Assay[1] Cell Line: Rat C6 glioma cells Concentration: 0 μM, 0.1 μM, 1 μM, 2 μM, 10 μM, 50 μM, 100 μM, 1000 μM Incubation Time: 0 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours Result: Inhibited cell viability of rat C6 glioma in vitro in a dose- and time-dependent manner. |
| In Vivo | Noscapine (300 mg/kg; oral gavage; daily; for 15 days; athymic female mice) treatment reduces tumor growth in mice[1]. Animal Model: Athymic female mice (nu/nu) (8-week-old) injected with rat C6 glioma cells[1] Dosage: 300 mg/kg Administration: Oral gavage; daily; for 15 days Result: Revealed a significant reduction of tumor volume. |
| References |
| Density | 1.332g/cm3 |
|---|---|
| Boiling Point | 565.3ºC at 760mmHg |
| Melting Point | 221-223ºC |
| Molecular Formula | C22H24ClNO7 |
| Molecular Weight | 449.882 |
| Flash Point | 295.7ºC |
| Exact Mass | 449.124115 |
| PSA | 75.69000 |
| LogP | 3.62170 |
| Storage condition | 2-8°C |
| Water Solubility | Freely soluble in water and in ethanol (96 per cent). Aqueous solutions are slightly acid; the base may be precipitated when the solutions are allowed to stand. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | UN 1544 |
| WGK Germany | 3 |
| RTECS | NP7225000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 0 | |
|---|---|
| DownStream 4 | |