51-35-4
| Name | 4-hydroxy-L-proline |
|---|---|
| Synonyms |
4-Hydroxyproline
(2S,4R)-4-hydroxypyrrolidinium-2-carboxylate Hydroxyproline MFCD00064320 L-Hydroxyproline trans-Lachnophyllol trans-4-hydroxyproline trans-L-4-hydroxyproline (2S,4R)-(-)-4-Hydroxypyrrolidine-2-carboxylic acid trans-4-Hydroxy-L-proline dec-2t-ene-4,6-diyn-1-ol L-trans-4-hydroxyproline (2S,4R)-4-Hydroxy-pyrrolidine-2-carboxylic acid L-Proline, 4-hydroxy-, trans- (9CI) Dec-2t-en-4,6-diin-1-ol EINECS 200-091-9 rans-4-Hydroxy-L-proline (-)-(2S,4R)-4-HYDROXYPROLINE (2S,4R)-4-hydroxyproline (2S,4R)-4-Hydroxypyrrolidine-2-carboxylic acid 4-Hydroxy-2-pyrrolidinecarboxylic acid (trans)-4-Hydroxy-L-proline (2S,4R)-trans-4-hydroxyproline (4R)-4-Hydroxy-L-proline (2S,4R)-4-Hydroxypyrrolidine-2-carboxylic acid,Hyp |
| Description | L-Hydroxyproline, one of the hydroxyproline (Hyp) isomers, is a useful chiral building block in the production of many pharmaceuticals. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | L-Hydroxyproline (Trans-4-hydroxy-L-proline;Trans-Hyp) has been widely used in medicine, biochemistry, food, cosmetic and other aspects of industry. Additionally, L-Hydroxyproline has also been found in the composition of some secondary metabolites such as actinomycins and echinocandins. L-Hydroxyproline is manufactured industrially most by acid hydrolysis of mammalian collagen because of its rich amount in the collagen[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 355.2±42.0 °C at 760 mmHg |
| Melting Point | 273 °C (dec.)(lit.) |
| Molecular Formula | C5H9NO3 |
| Molecular Weight | 131.130 |
| Flash Point | 168.6±27.9 °C |
| Exact Mass | 131.058243 |
| PSA | 69.56000 |
| LogP | -1.84 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.540 |
| Water Solubility | 357.8 g/L (20 º C) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TW3586500 |
| HS Code | 2933990090 |
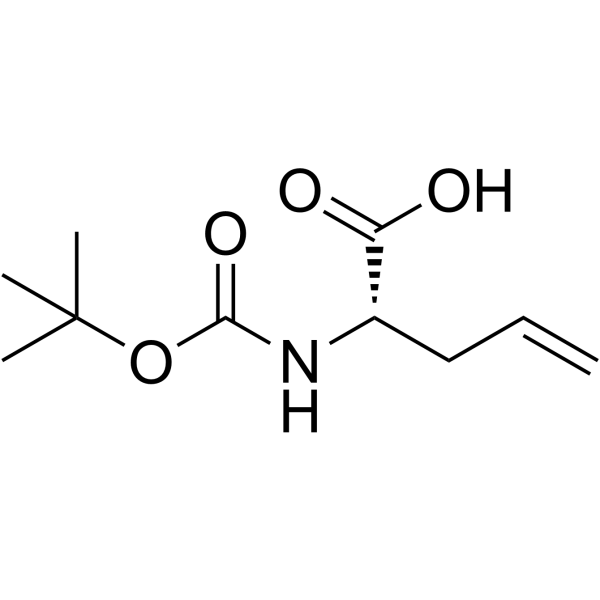
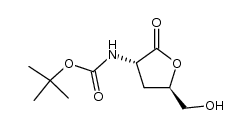
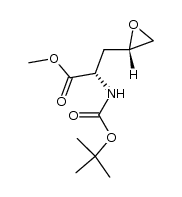
| Precursor 9 | |
|---|---|
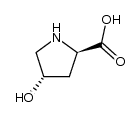
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![(2R,5S,7R)-2-(tert-butyl)-4-oxo-3-oxa-1-azabicyclo[3.3.0]oct-7-yl acetate structure](https://image.chemsrc.com/caspic/396/97652-00-1.png)
![1-[(3-nitrophenyl)methyl]pyrrole structure](https://image.chemsrc.com/caspic/317/107484-30-0.png)
![1-[(4-nitrophenyl)methyl]pyrrole structure](https://image.chemsrc.com/caspic/279/107484-31-1.png)