74844-91-0
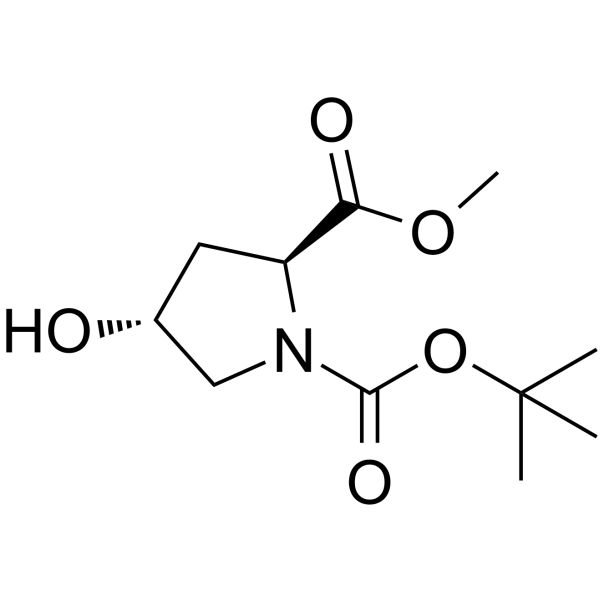
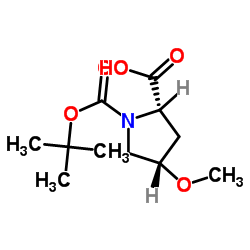
| Name | N-Boc-trans-4-Hydroxy-L-proline methyl ester |
|---|---|
| Synonyms |
(2S,4S)-4-Hydroxypyrrolidine-1,2-dicarboxylic Acid 1-tert-Butyl 2-Methyl Ester
N-BOC-transydro-L-prolinemethylester 1-O-tert-butyl 2-O-methyl (2S,4S)-4-hydroxypyrrolidine-1,2-dicarboxylate N-BOC-4-HYDROXY-L-PROLINE METHYL ESTER N-BOC-L-Hydroxyproline methyl ester 1-tert-Butyl 2-Methyl (2S,4S)-4-Hydroxypyrrolidine-1,2-dicarboxylate BOC-HYDROXYPROLINE-OME N-(tert-Butoxycarbonyl)-cis-4-hydroxy-L-proline Methyl Ester BOC-L-4-HYDROXYPROLINE METHYL ESTER N-Boc-trans-hydro-L-prolinemethylester N-Boc-cis-4-Hydroxy-L-prolinem ethylester N-Boc-trans-4-Hydroxy-L-prolinemethylester Boc-Hyp-Ome (2S,4S)-1-tert-Butyl 2-methyl 4-hydroxypyrrolidine-1,2-dicarboxylate MFCD00076981 BOC-L-HYDROXYPROLINE METHYL ESTER BOC-L-T-4-HYDROXYPROLINE METHYL ESTER Boc-trans-4-hydroxy-L-proline methyl ester N-Boc-cis-4-hydroxy-L-proline methyl ester Boc-cis-Hyp-OMe cis-Boc-Hyp-OMe |
| Description | Boc-Hyp-OMe is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). Boc-Hyp-OMe is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Non-cleavable |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 335.2±42.0 °C at 760 mmHg |
| Melting Point | 92-96 °C(lit.) |
| Molecular Formula | C11H19NO5 |
| Molecular Weight | 245.272 |
| Flash Point | 156.6±27.9 °C |
| Exact Mass | 245.126328 |
| PSA | 76.07000 |
| LogP | -0.25 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.501 |
| Storage condition | 2~8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |