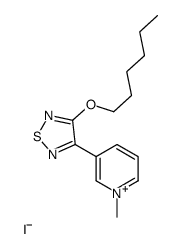
141064-23-5
| Name | 3-hexoxy-4-(1-methyl-3,6-dihydro-2H-pyridin-5-yl)-1,2,5-thiadiazole,oxalic acid |
|---|---|
| Synonyms |
xanomeline oxalate
Pyridine, 3-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1,2,5,6-tetrahydro-1-methyl-, ethanedioate (1:1) 5-[4-(Hexyloxy)-1,2,5-thiadiazol-3-yl]-1-methyl-1,2,3,6-tetrahydropyridine ethanedioate (1:1) unii-jf5a0pk3g5 Xanomeline (oxalate) |
| Description | Xanomeline(LY246708) is a selective M1 muscarinic receptor agonist.IC50 value:Target: M1 muscarinic receptorin vitro: Xanomeline had high affinity for muscarinic receptors in brain homogenates, but had substantially less or no affinity for a number of other neurotransmitter receptors and uptake sites. In cells stably expressing genetic m1 receptors, xanomeline increased phospholipid hydrolysis in CHO, BHK and A9 L cells to 100, 72 and 55% of the nonselective agonist carbachol. In isolated tissues, xanomeline had high affinity for M1 receptors in the rabbit vas deferens (IC50 = 0.006 nM), low affinity for M2 receptors in guinea pig atria (EC50 = 3 microM), was a weak partial agonist in guinea pig ileum and was neither an agonist nor antagonist in guinea pig bladder [1]. Xanomeline produced small increases in striatal acetylcholine levels and did not antagonize the large increases in acetylcholine produced by the nonselective muscarinic agonist oxotremorine, indicating that xanomeline did not block M2 autoreceptors [2]. in vivo: Xanomeline increased striatal levels of dopamine metabolites, presumably by acting at M1 heteroreceptors on dopamine neurons to increase dopamine release. In contrast, xanomeline had only a relatively small effect on acetylcholine levels in brain, indicating that it is devoid of actions at muscarinic autoreceptors [1]. The effects of xanomeline on ex vivo binding and DOPAC levels lasted for about 3 hr and were evident after oral administration. An analog of xanomeline with similar in vivo effects did not inhibit acetylcholinesterase or choline acetyltransferase and inhibited choline uptake only at concentrations much higher than those required to inhibit binding. These data indicate xanomeline is selective agonist for M1 over M2 and M3 receptors in vivo in rat [2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C16H25N3O5S |
|---|---|
| Molecular Weight | 371.452 |
| Exact Mass | 371.151489 |
| PSA | 141.09000 |
| LogP | 2.30960 |
| Storage condition | 2-8℃ |
|
~% 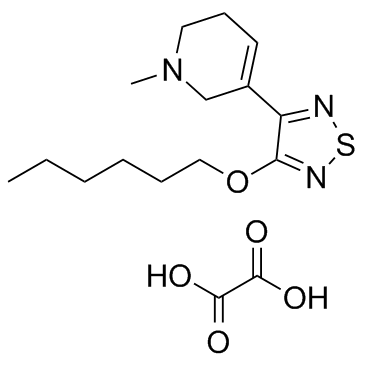
141064-23-5 |
| Literature: Novo Nordisk A/S Patent: US5545638 A1, 1996 ; US 5545638 A Title/Abstract Full Text Show Details Novo Nordisk A/S Patent: US5750541 A1, 1998 ; US 5750541 A |
| Precursor 1 | |
|---|---|
| DownStream 0 | |