743461-65-6
| Name | batefenterol |
|---|---|
| Synonyms |
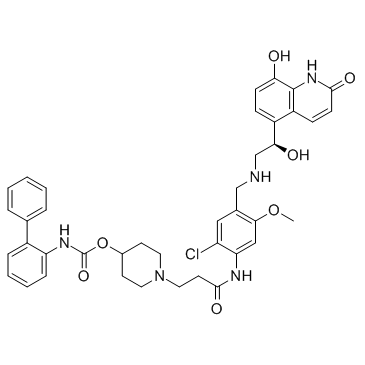
Carbamic acid, N-[1,1'-biphenyl]-2-yl-, 1-[3-[[2-chloro-4-[[[(2R)-2-(1,2-dihydro-8-hydroxy-2-oxo-5-quinolinyl)-2-hydroxyethyl]amino]methyl]-5-methoxyphenyl]amino]-3-oxopropyl]-4-piperidinyl ester
1-(3-{[2-Chloro-4-({[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro-5-quinolinyl)ethyl]amino}methyl)-5-methoxyphenyl]amino}-3-oxopropyl)-4-piperidinyl 2-biphenylylcarbamate GSK961081A 1-(3-{[2-chloro-4-({[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo- 1,2-dihydroquinolin-5-yl)ethyl]amino}methyl)- 5-methoxyphenyl]amino}-3-oxopropyl)piperidin-4-yl (1,1-biphenyl- 2-yl)carbamate batefenterol GSK-961081 |
| Description | Batefenterol (GSK961081;TD-5959) is a novel muscarinic receptor antagonist and β2-adrenoceptor agonist; displays high affinity for hM2, hM3 muscarinic and hβ2-adrenoceptor with Ki values of 1.4, 1.3 and 3.7 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 1.4 nM (hM2), 1.3 nM (hM3), 3.7 (β2)[1] |
| In Vitro | Batefenterol is a novel first-in-class inhaled bifunctional compound possessing both muscarinic antagonist (MA) and β2-adrenoceptor agonist (BA) properties (MABA). In competition radioligand binding studies at human recombinant receptors, batefenterol displays high affinity for hM2 (Ki=1.4 nM), hM3 muscarinic receptors (Ki=1.3 nM) and hβ2-adrenoceptors (Ki=3.7 nM). Batefenterol behaves as a potent hβ2-adrenoceptor agonist (EC50=0.29 nM for stimulation of cAMP levels) with 440- and 320-fold functional selectivity over hβ1- and hβ3-adrenoceptors, respectively[1]. |
| In Vivo | In the guinea pig bronchoprotection assay, inhaled Batefenterol produces potent, dose-dependent inhibition of bronchoconstrictor responses via MA (ED50=33.9 µg/mL), BA (ED50=14.1 µg/mL), and MABA (ED50=6.4 µg/mL) mechanisms. Significant bronchoprotective effects of Batefenterol are evident in guinea pigs via MA, BA, and MABA mechanisms for up to 7 days after dosing[1]. In guinea pig isolated trachea expressing native muscarinic M3 and β2, batefenterol produces smooth muscle relaxation through a dual mechanism involving competitive antagonism of the M3 receptor (EC50=50 nM) and agonism of the β2 receptor (EC50=25 nM). The combined effect on both muscarinic receptors and β2 receptors is more potent than either function working alone (EC50=10 nM). Batefenterol exhibits a rapid rate of clearance and short half-life[2]. |
| Cell Assay | CHO-K1 cells stably transfected with each receptor subtype are incubated with increasing concentrations of batefenterol for 20 minutes at 37°C. The cells are stimulated with an EC90 concentration of the muscarinic agonist oxotremorine. Oxotremorine elicits a Gq-mediated calcium-release event, which in turn caused the calcium-sensitive dye to bind to calcium and fluoresce upon stimulation with a 488 nm laser light source. The change in fluorescence is measured by the FLIPR for 3 minutes, and the peak height in fluorescence is taken as the maximal response to generate the concentration-response curve for batefenterol[1]. |
| Animal Admin | Guinea pigs: Batefenterol is dissolved in water. Guinea pig trachea is dissected and isolated. The tracheal rings are initially tensioned to 1 g and allowed to equilibrate for 1 hour before evoking contraction with a submaximal concentration of either methylcholine (MCh; 10 µM), in the presence of propranolol (10 µM), or histamine (HIS; 30 µM) to assess relaxant effects via MA and BA mechanisms, respectively. Relaxation through the MABA mechanism is evaluated in tissues precontracted with MCh in the absence of propranolol. After the contractile tone attained a plateau, the batefenterol (0.1 nM to 100 µM) is added cumulatively in half log increments, with each concentration being added after achieving a steady-state relaxation response to the previous concentration. After the last concentration of test compound, theophylline (2.2 mM) is added to establish maximum relaxation[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 948.3±65.0 °C at 760 mmHg |
| Molecular Formula | C40H42ClN5O7 |
| Molecular Weight | 740.244 |
| Flash Point | 527.3±34.3 °C |
| Exact Mass | 739.277283 |
| LogP | 5.26 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | 2-8℃ |