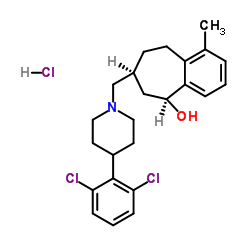
371980-94-8
| Name | (5S,7S)-7-{[4-(2,6-Dichlorophenyl)-1-piperidinyl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol hydrochloride (1:1) |
|---|---|
| Synonyms |
MFCD12546333
5H-Benzocyclohepten-5-ol, 7-[[4-(2,6-dichlorophenyl)-1-piperidinyl]methyl]-6,7,8,9-tetrahydro-1-methyl-, (5S,7S)-, hydrochloride (1:1) (5S,7S)-7-{[4-(2,6-Dichlorophenyl)-1-piperidinyl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol hydrochloride (1:1) SB-612111 hydrochloride |
| Description | SB-612111 hydrochloride is a novel and potent human opiate receptor-like orphan receptor (ORL-1) antagonist with a high affinity for hORL-1 (Ki=0.33 nM). SB-612111 hydrochloride exhibits selectivity for μ-, κ- and δ-receptors with Ki values of 57.6 nM, 160.5 nM and 2109 nM, respecticely. SB-612111 hydrochloride effectively antagonizes the pronociceptive action of Nociceptin (HY-P0183) in an acute pain model[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.33 nM (hORL-1) Ki: 57.6 nM (μ-receptor); 160.5 nM (κ-receptor); 2109 nM (δ-receptor)[1] |
| In Vivo | SB-612111 hydrochloride (intravenous injection; 0.6-10 nmol/mouse) antagonize nociceptin-induced thermal hyperalgesia in a dose-dependent manner with an ED50 of 0.62 mg/kg[1]. SB-612111 hydrochloride (intravenous injection; 0.1-5 mg/kg) causes a significant inhibition of the carrageenan-induced reduction in paw withdrawal latencies in rat, however, untreated paw are uneffected[1]. Animal Model: Male rats[1] Dosage: 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 5 mg/kg Administration: Intravenous injection; single dose Result: Had antihyperalgesic effects on carrageenan-induced rat paw. |
| References |
| Molecular Formula | C24H30Cl3NO |
|---|---|
| Molecular Weight | 454.860 |
| Exact Mass | 453.139313 |