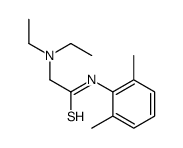
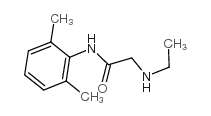
137-58-6
| Name | lidocaine |
|---|---|
| Synonyms |
2-Diethylamino-N-(2,6-dimethylphenyl)acetamide
Lignocaine 4,4'-DIMETHOXY DIPHENYL SULFOXIDE LIDOCAINE-D6 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide,Lignocaine,Xylocaine Maricaine MFCD08443609 Gravocain 2-(Diethylamino)-N-(2,6-dimethylphenyl)-acetamide Lidoderm N1-(2,6-dimethylphenyl)-2-(diethylamino)acetamide 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide XYLOCAINE N-(2,6-Dimethylphenyl)-N,N-diethylglycinamide 2-Diethylamino-2',6'-acetoxylidide N-(2,6-Diméthylphényl)-N,N-diéthylglycinamide Anestacon L-Caine Leostesin Esracaine Lidothesin 2-Diethylamino-N-(2,6-dimethylphenyl)acetamide Lignocaine Xylocaine N-(2,6-dimethylphenyl)-N2,N2-diethylglycinamide lignocainum hydrochloricum Lidocaine w-Diethylamino-2,6-dimethylacetanilide EINECS 205-302-8 |
| Description | Lidocaine, an amide local anesthetic, has anti-inflammatory properties in vitro and in vivo, possibly due to an attenuation of pro-inflammatory cytokines, intracellular adhesion molecule-1 (ICAM-1), and reduction of neutrophils influx.Target: Lidocaine is a common local anesthetic and antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning and pain from skin inflammations, injected as a dental anesthetic or as a local anesthetic for minor surgery. Lidocaine, the first amino amide–type local anesthetic, was first synthesized under the name xylocaine by Swedish chemist Nils Lofgren in 1943. His colleague Bengt Lundqvist performed the first injection anesthesia experiments on himself.Lidocaine is approximately 95% metabolized (dealkylated) in the liver by CYP3A4 to the pharmacologically-active metabolites monoethylglycinexylidide (MEGX) and then subsequently to the inactive glycine xylidide. MEGX has a longer half life than lidocaine but also is a less potent sodium channel blocker. The elimination half-life of lidocaine is approximately 90–120 minutes in most patients. This may be prolonged in patients with hepatic impairment (average 343 minutes) or congestive heart failure (average 136 minutes). |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 372.7±52.0 °C at 760 mmHg |
| Melting Point | 66-69°C |
| Molecular Formula | C14H22N2O |
| Molecular Weight | 234.337 |
| Flash Point | 179.2±30.7 °C |
| Exact Mass | 234.173218 |
| PSA | 32.34000 |
| LogP | 3.63 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.512 |
| Storage condition | Store at RT |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | practically insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22-S26-S36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | AN7525000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2924299090 |
|
~% 
137-58-6 |
| Literature: Arkiv foer Kemi, , vol. 22A, # 18 p. 11 |
|
~% 
137-58-6 |
| Literature: Arkiv foer Kemi, , vol. 22A, # 18 p. 11 |
|
~% 
137-58-6 |
| Literature: Langmuir, , vol. 26, # 13 p. 10561 - 10571 |
| Precursor 3 | |
|---|---|
| DownStream 9 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |