111-58-0
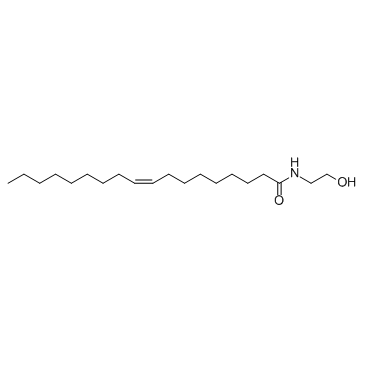
| Name | oleoyl ethanolamide |
|---|---|
| Synonyms |
Oleic acid ethanolamide
MFCD08059579 N-(2-Hydroxyethyl)oleamide N-(Hydroxyethyl)oleamide N-(cis-9-Octadecenoyl)ethanolamine OEA oleoylethanolamide N-(9Z-octadecenoyl)-ethanolamine Oleoyl monoethanolamide (9Z)-N-(2-hydroxyethyl)octadec-9-enamide N-oleoyl ethanolamide Oleoylethanolamide N-Oleoylethanolamine N-Oleoyl-2-aminoethanol (9Z)-N-(2-Hydroxyethyl)-9-octadecenamide N-(cis-9-Octadecenoyl)ethanolamine N-oleoyl ethanolamine Oleic acid monoethanolamide EINECS 203-884-8 9Z-octadecenoylethanolamide Oleoyl Ethanolamide |
| Description | Oleoylethanolamide is a high affinity endogenous PPAR-α agonist, which plays an important role in the treatment of obesity and arteriosclerosis. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite PPAR-α |
| In Vitro | Oleoylethanolamide (OEA), an endogenous PPAR-α ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oleoylethanolamide suppresses TGF-β1 induced hepatic stellate cells (HSCs) activation in vitro via PPAR-α. To assess the impact of Oleoylethanolamide on HSCs activation, the expression levels of α-SMA and Col1a in TGF-β1-stimulated HSCs are examined by qPCR. The mRNA levels of α-SMA and Col1a are markedly induced in the group of CFSC cells with TGF-β1 (5 ng/mL) stimulation for 48h, while the mRNA levels are suppressed when treated with Oleoylethanolamide in a dose-dependent manner. Immunofluorescence and western blot results show that Oleoylethanolamide treatment dose-dependently inhibits the protein expression of α-SMA, the marker of HSC activation. The inhibitory effects of Oleoylethanolamide on HSCs activation are completely blocked by PPAR-α antagonist MK886 (10 μM). Moreover, the mRNA and protein expression levels of PPAR-α are down-regulated with TGF-β1 stimulation, while Oleoylethanolamide treatment restores these changes in dose-dependent manner. In addition, the phosphorylation of Smad 2/3 is upregulated in the presence of TGF-β1 stimulation, consistent with the observed effects on HSC activation, while Oleoylethanolamide (10 μM) reduces the phosphorylation of Smad2/3 in CFSC simulated with TGF-β1[1]. |
| In Vivo | Oleoylethanolamide (OEA) can significantly suppress the pro-fibrotic cytokine TGF-β1 negatively regulate genes in the TGF-β1 signaling pathway (α-SMA, collagen 1a, and collagen 3a) in mice models of hepatic fibrosis. Treatment with Oleoylethanolamide (5 mg/kg/day, intraperitoneal injection, i.p.) significantly attenuates the progress of liver fibrosis in both two experimental animal models by blocking the activation of hepatic stellate cells (HSCs)[1]. |
| Cell Assay | CFSC, HSC cell lines are first obtained from cirrhotic rat liver, and have a similar phenotype to that of early passage primary HSCs. CFSC cells are cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. All cells are cultured in 6-well culture plates under 37°C and 5% CO2 in an incubator. The medium is replaced every two days, and the cells are harvested and diluted at a ratio of 1:3 twice a week. In experiments, HSCs are pretreated with the experimental concentration of Oleoylethanolamide (30 μM, 10 μM, 3 μM) before stimulation with 5 ng/mL TGF-β1. mRNA expression levels of α-SMA (A) and Col1a (B) are analyzed by real-time PCR[1]. |
| Animal Admin | Mice[1] The Sv/129 mice and PPAR-α knockout mice are maintained in a room with controlled temperature (21-23°C), humidity (55-60%) and lighting (12 h light/dark cycles) and given water ad libitum. Mice are randomly divided for methionine choline-deficient (MCD) and thioacetamide (TAA) experiments. In the MCD-diet feeding experiment, wild-type Sv/129 mice and PPAR-α knockout mice are each divided into three groups (n=8 /group): (i) control group receive normal diet; (ii) fed with MCD diet and injected with the vehicle (5% Tween-80+5% PEG400+90% saline, 5 mL/kg/day, 8 weeks, intraperitoneal injection, i.p.); (iii) fed with MCD diet along with Oleoylethanolamide administration (5 mg/kg/day; 8 weeks, i.p.). In another set of experiment, all the wild-type mice and PPAR-α knockout mice are given standard chow diet, and are randomly separated into three groups: the control group is not administrated TAA or Oleoylethanolamide but is injected with the saline; the TAA group is injected with TAA (160 mg/kg, three times per week, 6 weeks, dissolved in saline, i.p.) plus the corresponding vehicle; the Oleoylethanolamide group is both injected with TAA and Oleoylethanolamide (5 mg/kg/day; 6 weeks, i.p.)[1]. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 496.4±38.0 °C at 760 mmHg |
| Melting Point | 50-60ºC |
| Molecular Formula | C20H39NO2 |
| Molecular Weight | 325.529 |
| Flash Point | 254.0±26.8 °C |
| Exact Mass | 325.298065 |
| PSA | 49.33000 |
| LogP | 6.36 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.474 |
| Storage condition | −20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |