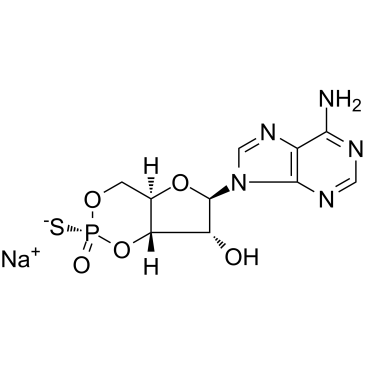
142439-94-9
| Name | Rp-cAMPS sodium salt |
|---|
| Description | Rp-cAMPS sodium salt, a cAMP analog, is a potent, competitive cAMP-induced activation of cAMP-dependent PKA I and II (Kis of 12.5 µM and 4.5 µM, respectively) antagonist. Rp-cAMPS sodium salt is resistant to hydrolysis by phosphodiesterases[1][2][3][4][5][6]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 6.05 µM (PKA I) and 9.75 µM (PKA II)[1] |
| In Vitro | A membrane-permeable competitive cAMP antagonist (Rp-cAMPS) that blocks PKA activation by binding to the regulatory subunits without dissociating the kinase holoenzyme also inhibits synaptic plasticity but has no effect on normal synaptic transmission[2]. |
| In Vivo | Rp-cAMPS (10 μM, 15 min) decreases the monosynaptic EPSCs evoked at the PB-CeLC and BLA-CeLC synapses in slices from arthritic rats but not in control neurons from normal animals. The inhibitory effect of Rp-cAMPS is significant compared to predrug (ACSF) control values obtained in the same neurons[2]. |
| References |
| Molecular Formula | C10H11N5NaO5PS |
|---|---|
| Molecular Weight | 367.25 |