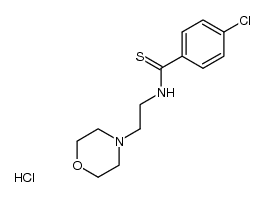
71320-77-9
| Name | moclobemide |
|---|---|
| Synonyms |
4-chloro-N-[2-(morpholin-4-yl)ethyl]benzamide
Moclamide moclbemide Manefix Moclobemide aurorix 4-Chloro-N-(2-(4-morpholinyl)ethyl)benzamide 4-Chloro-N-[2-(4-morpholinyl)ethyl]benzamide MFCD00865388 Moclobemidum MODOBEMDE ro11-1163 Moclaime p-Chloro-N-(2-morpholinoethyl)benzamide UNII-PJ0Y7AZB63 manerix Moclamine |
| Description | Moclobemide(Ro111163) is a reversible monoamine oxidase inhibitor (MAOI) selective for isoform A (RIMA) used to treat major depressive disorder.Target: Monoamine OxidaseMoclobemide orally administered 2 hours before decapitation preferentially inhibits MAO-A and PEA in rat brain with ED50 of 7.6 μmol/kg and 78 μmol/kg, respectively. Moclobemide orally administered 2 hours before decapitation preferentially inhibits MAO-A and PEA in rat liver with ED50 of 8.4 μmol/kg and 6.6 μmol/kg, respectively. Moclobemide (0.1 mM), which inhibits brain MAO-A activity by over 80%, does not affect benzylamine oxidase (rat heart) and diamine oxidase (rat small intestine) activity in vitro [1]. Moclobemide (10 mM-100 mM) includes in the culture medium during anoxia or with glutamate significantly increases in a concentration-dependent manner the amount of surviving neurons compared to controls in neuronal-astroglial cultures from rat cerebral cortex [2].Moclobemide (10 mg/kg p.o.) induces a significant decrease of all monoamine metabolites measured in rat brain [1]. Moclobemide, given via the drinking water (4.5 mg/kg/day), produces significant decreases in adrenal weight of rats after 5 (-23%) and 7 weeks (-16%) of treatment. Moclobemide upregulates hippocampal mineralocorticoid receptor (MR) levels in rats by 65%, 76% and 19% at 2 weeks, 5 weeks and 7 weeks of treatment, and upregulates Glucocorticoid receptor (GR) levels in this limbic brain structure by 10% at 5 weeks. Moclobemide treatment (5 weeks, 4.5 mg/kg/day) significantly attenuates stress (30 min novel environment)-induced plasma ACTH (-35%) and corticosterone (-29%) levels [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 447.7±40.0 °C at 760 mmHg |
| Melting Point | 137°C |
| Molecular Formula | C13H17ClN2O2 |
| Molecular Weight | 268.739 |
| Flash Point | 224.6±27.3 °C |
| Exact Mass | 268.097870 |
| PSA | 41.57000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.550 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S26-S39 |
| RIDADR | 3249 |
| RTECS | CV2462000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 9 | |
|---|---|
| DownStream 0 | |