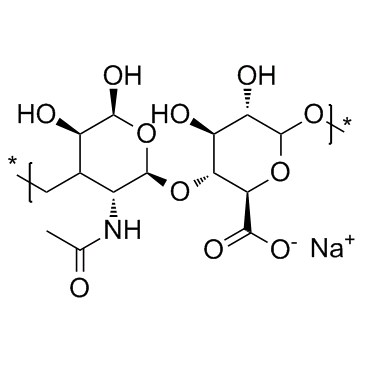
9004-61-9
| Name | hyaluronic acid |
|---|---|
| Synonyms |
(4ξ)-α-D-xylo-hexopyranuronosyl-(1->;3)-2-(acetylamino)-2-deoxy-β-D-glucopyranosyl-(1->4)-(5ξ)-β-D-xylo-hexopyranuronosyl-(1->;3)-2-(acetylamino)-2-deoxy-D-glucopyranose
(4ξ)-α-D-xylo-Hexopyranuronosyl-(1->;3)-2-acetamido-2-deoxy-β-D-allopyranosyl-(1->4)-(5ξ)-β-D-xylo-hexopyranuronosyl-(1->3)-2-acetamido-2-deoxy-D-glucopyranose Hyaluronic Acid Sodium Salt MFCD00131348 EINECS 232-678-0 |
| Description | Hyaluronic acid is a biopolymer composed of repeating units of disaccharides with various applications. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Hyaluronic acid (HA) is widely used in aesthetic medicine due to its binding ability with a large number of water molecules. It improves tissue hydration and their resistance to mechanical damage. HA plays an important role in wound healing, ovulation, fertilization, signal transduction, and tumor physiology. HA is used in joint diseases such as osteoarthritis or rheumatoid arthritis. HA of a high molecular mass reduces the chemotaxis and migration of inflammatory cells which acts as a good barrier to the inflammatory process and protects against the effects of free radicals. HA is used in ophthalmology due to its lubricating properties for the corneal endothelium, and improves tissue hydration and cellular resistance to mechanical damage in aesthetic dermatology, and has marginal adverse effects. Several trials indicate its role in tumor markers, liver diseases, and in pharmaceuticals[1]. Hyaluronan plays an important role in cancer growth and metastasis. HA and HA fragment-tumor cell interaction could activate the downstream signaling pathways, promoting cell proliferation, adhesion, migration and invasion, and inducing angiogenesis, lymphangiogenesis, epithelial-mesenchymal transition, stem cell-like property, and chemoradioresistance in digestive cancers[2]. |
| In Vivo | The impact of applied intra-articular HA has been proven in many studies in animals. Studies on HA have shown that it promotes the synthesis of cartilage matrix, prevents its degradation, reduces inflammation, stimulates the synthesis of endogenous HA, and improves the resilience and moisture of cartilage [1]. High molecular size HA preparations, applied topically, promote healing of fresh skin wounds. They also promote the healing of venous leg ulcers and are useful in the management of chronic wounds[3]. |
| References |
[2]. Wu RL, et al. Hyaluronic acid in digestive cancers. J Cancer Res Clin Oncol. 2017 Jan;143(1):1-16. |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 1274.4±65.0 °C at 760 mmHg |
| Molecular Formula | (C14H21NO11)n |
| Molecular Weight | 379.32 (monomer) |
| Flash Point | 724.5±34.3 °C |
| PSA | 399.71000 |
| LogP | -6.62 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.666 |
| Storage condition | −20°C |
| Water Solubility | H2O: 5 mg/mL, clear, colorless | Soluble in water. |
|
~% 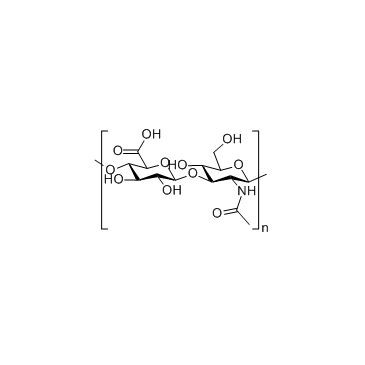
9004-61-9 |
| Literature: US2006/172967 A1, ; Page/Page column 5 ; |
| HS Code | 3004909090 |
|---|