36011-19-5
| Name | cytochalasin e |
|---|---|
| Synonyms |
MFCD00005178
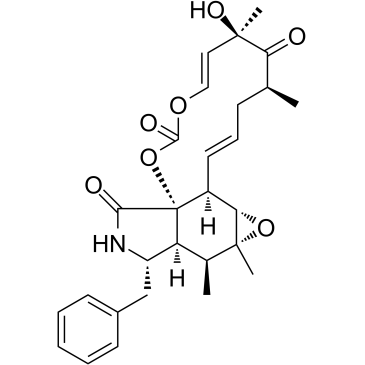
EINECS 252-835-7 (1Z)-14-Benzyl-6-hydroxy-4,6,15,15a-tetramethyl-3,13,14,14a,15,15a,16a,16b-octahydro[1,3]dioxacyclotridecino[4,5-d]oxireno[f]isoindole-5,10,12(4H,6H)-trione (1Z,7E)-14-Benzyl-6-hydroxy-4,6,15,15a-tetramethyl-3,13,14,14a,15,15a,16a,16b-octahydro[1,3]dioxacyclotridecino[4,5-d]oxireno[f]isoindole-5,10,12(4H,6H)-trione CYTOCHALACINE CYTOCHALASIN 3 CYTOCHALICINE CYTOCHALASIN E,ASPERGILLUS CLAVATUS (7s,13e,16s,18r,19e)-18-dimethyl-10-phenyl |
| Description | Cytochalasin E, an epoxide containing Aspergillus-derived fungal metabolite, inhibits angiogenesis and tumor growth. Cytochalasin E is a potent actin depolymerization agent, and it binds and caps the barbed end of actin filaments to prevent actin elongation[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cytochalasin E prominently inhibits the growth of A549 cells in a dose-dependent manner[3]. Cytochalasin E could induce the up-regulation of autophagy-related protein (LC3-II) and SQSTM1/p62[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 705.1±60.0 °C at 760 mmHg |
| Melting Point | 206ºC |
| Molecular Formula | C28H33NO7 |
| Molecular Weight | 495.564 |
| Flash Point | 380.2±32.9 °C |
| Exact Mass | 495.225708 |
| PSA | 114.46000 |
| LogP | 1.92 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.608 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330-H361 |
| Precautionary Statements | P260-P264-P280-P284-P301 + P310-P302 + P350 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+,T |
| Risk Phrases | R26/27/28 |
| Safety Phrases | 28-36/37-45 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | HA5360000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 29349990 |