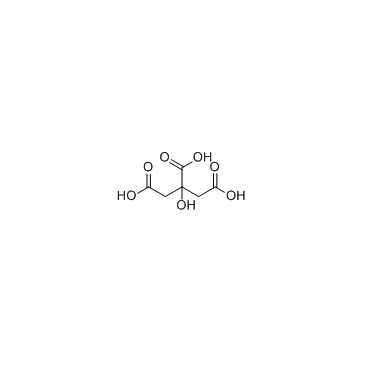
3458-72-8
| Name | triammonium citrate |
|---|---|
| Synonyms |
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, triammonium salt
CITRIC ACID DIAMMONIUM SALT 2-Hydroxy-1,2,3-propanetricarboxylic acid triammonium salt Triammonium citrate 99-1 Tri-AmmoniumCitrateGr AMMONIUM HYDROGENCITRATE Ammonium citrate tribasic 2-Hydroxy-1,2,3-prop MFCD00013068 Citric acid triammonium salt tri-ammonium citrate citric acid,triammonium compound Tri-Ammoniumcitrat CITRIC ACID,DIAMMONIUM Tri-AmmoniumCitrateExtraPure ammonium citrate Citronensaeure,Triammonium-Verbindung Triammonium citrate Citric Acid,Triammonium Salt EINECS 222-394-5 |
| Description | Citric acid triammonium (Triammonium citrate) is formed by Citric acid (HY-N1428) reacting with ammonia in a molar ratio of 1:3. Citric acid triammonium can be used as the carbon source to prepare carbon quantum dots (CDs). Citric acid triammonium with higher nitrogen components might promote the nitrogen-based functional groups in CDs, leading to a more efficient emission-color tunability[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.22 g/mL at 20 °C |
|---|---|
| Boiling Point | 100 °C(lit.) |
| Melting Point | 185 °C (dec.)(lit.) |
| Molecular Formula | C6H17N3O7 |
| Molecular Weight | 243.215 |
| Flash Point | 155.2ºC |
| Exact Mass | 243.106644 |
| PSA | 141.85000 |
| Vapour density | 1.8 (vs air) |
| Index of Refraction | 1.583 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | H2O: 1 M at 20 °C, clear, colorless |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GE7545000 |
|
~% 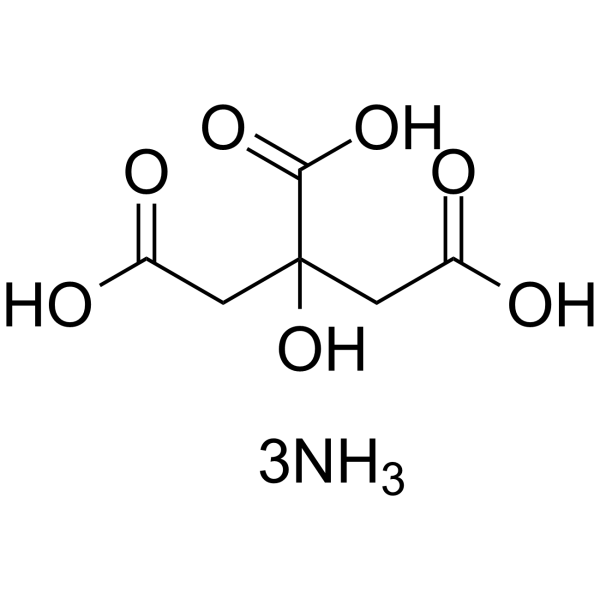
3458-72-8 |
| Literature: Journal of the American Chemical Society, , vol. 37, p. 211 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |