65085-01-0
| Name | cefmenoxime |
|---|---|
| Synonyms |
Cefmenoxime
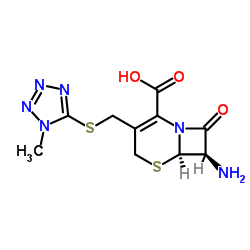
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-,(6R,7R) (6R,7R)-7-[[(Z)-(2-Aminothiazol-4-yl)(methoxyimino)acetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid SCE 1365 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido]-3-[[1-methyl-1H-tetrazol-5-yl]thiomethyl]-3-cephem-4-carboxylic acid MFCD00864851 CMX |
| Description | Cefmenoxime (SCE-1365) is a new semisynthetic cephalosporin antibiotic. Cefmenoxime has antibacterial activity against a wide variety of gram-positive and gram-negative bacteria[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cefmenoxime (SCE-1365) inhibits at least 90% of strains tested (MIC90) ranged from 0.06 to 8 μg/mL for the Enterobacteriaceae[1]. Cefmenoxime (SCE-1365) inhibits MIC90 values for gram-positive cocci are 0.015 and ≤0.008 μg/mL for Streptococcus pneumoniae and Streptococcus pyogenes, respectively, and 2 μg/mL for S. aureus[1]. Cefmenoxime (SCE-1365) against Haemophilus influenzae, Neisseria gonorrhoeae and Neisseria meningitidis with MIC90 values ranging from ≤0.008 to 0.25 μg/mL[1]. |
| In Vivo | Cefmenoxime (SCE-1365) (40 mg/kg; i.h.; daily, for 7 d; male Jcl:ICR mice) improves the survival rate of mice infected with lung bacteria[2]. Animal Model: Male Jcl:ICR mice[2] Dosage: 40 mg/kg Administration: Subcutaneous injection; daily, for 7 days Result: Inhibited mortality rate of animals was 60% at a dose of 40 mg/kg. |
| References |
| Density | 1.96 g/cm3 |
|---|---|
| Molecular Formula | C16H17N9O5S3 |
| Molecular Weight | 511.55800 |
| Exact Mass | 511.05100 |
| PSA | 269.65000 |
| LogP | 0.04020 |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R41:Risk of serious damage to eyes. |
| Safety Phrases | S26-S39 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |