68353-24-2
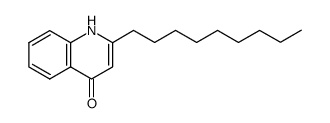
| Name | 1-methyl-2-nonylquinolin-4-one |
|---|---|
| Synonyms |
1-Methyl-2-nonyl-4(1H)-quinolinone
N-methyl-2-nonyl-4-quinolone 1-Methyl-2-nonyl-4(1H)-quinolone 1-Methyl-2-n-nonyl-4-chinolon 1-methyl-2-nonyl-1H-quinolin-4-one |
| Description | 1-Methyl-2-nonyl-4(1H)-quinolone, a quinolone alkaloid, is a potent and selective MAO-B (monoamine oxidase) inhibitor. 1-Methyl-2-nonyl-4(1H)-quinolone exhibites inhibitory activity on leukotriene biosynthesis, with an IC50 of 12.1 μM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MAO-B MAO-A |
| In Vitro | 1-Methyl-2-nonyl-4(1H)-quinolone shows more potent inhibitory effects against MAO-B compared to MAO-A[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 391.9±42.0 °C at 760 mmHg |
| Melting Point | 73-75℃ |
| Molecular Formula | C19H27NO |
| Molecular Weight | 285.424 |
| Flash Point | 132.6±17.3 °C |
| Exact Mass | 285.209259 |
| PSA | 22.00000 |
| LogP | 6.09 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.524 |
| Hazard Codes | Xi |
|---|
|
~10% 
68353-24-2 |
| Literature: Somanathan; Smith Journal of Heterocyclic Chemistry, 1981 , vol. 18, # 6 p. 1077 - 1079 |
|
~% 
68353-24-2 |
| Literature: Somanathan; Smith Journal of Heterocyclic Chemistry, 1981 , vol. 18, # 6 p. 1077 - 1079 |
|
~% 
68353-24-2 |
| Literature: Somanathan; Smith Journal of Heterocyclic Chemistry, 1981 , vol. 18, # 6 p. 1077 - 1079 |
|
~% 
68353-24-2 |
| Literature: Somanathan; Smith Journal of Heterocyclic Chemistry, 1981 , vol. 18, # 6 p. 1077 - 1079 |
|
~% 
68353-24-2 |
| Literature: Somanathan; Smith Journal of Heterocyclic Chemistry, 1981 , vol. 18, # 6 p. 1077 - 1079 |
|
~% 
68353-24-2 |
| Literature: Coppola, Gary M. Journal of Heterocyclic Chemistry, 1985 , vol. 22, p. 491 - 494 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |