2415-24-9
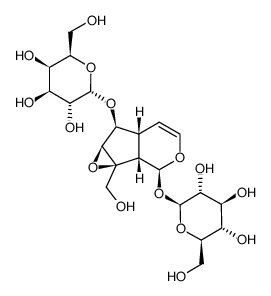
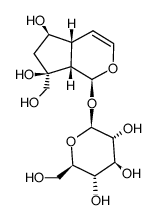
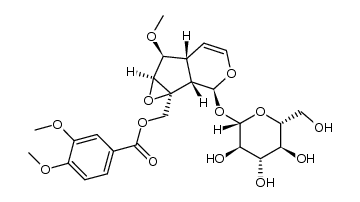
| Name | Catalpol |
|---|---|
| Synonyms |
EINECS 219-324-0
catalpinoside des-p-hydroxybenzoyl-catalposid CATAPOL 6-Hydroxy-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-2-yl hexopyranoside Hexopyranoside, 1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl catalposide methyl iridoid glycoside |
| Description | Catalpol, an iridoid glycoside, has neuroprotective, anti-inflammatory, and anti-hepatitis virus effects.IC50 Value:Target: neuroprotective, anti-inflammatory, and anti-hepatitis virus natural product.In vitro: Catalpol could be encapsulated into composite nanofibers and induce differentiation of hASCs into neural-like cells, which might offer new avenues in nerve regeneration [1].In vivo: The pharmacokinetics of catalpol in normal and doxorubicin-induced chronic kidney disease rats after oral administration of Rehmannia glutinosa extract was determined, and the extraction recoverie of catalpol was higher than 68.24% [2]. The protective effect of catalpol on renal IRI mice through suppressing phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-endothelial nitric oxide synthase (eNOS) and against inflammation, and the possible underlying mechanism [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 675.6±55.0 °C at 760 mmHg |
| Melting Point | 203-205ºC |
| Molecular Formula | C15H22O10 |
| Molecular Weight | 362.329 |
| Flash Point | 362.4±31.5 °C |
| Exact Mass | 362.121307 |
| PSA | 161.60000 |
| LogP | -4.15 |
| Vapour Pressure | 0.0±4.7 mmHg at 25°C |
| Index of Refraction | 1.679 |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| RTECS | LZ5776816 |
|
~% 
2415-24-9 |
| Literature: Oshio,H.; Inouye,H. Phytochemistry (Elsevier), 1982 , vol. 21, p. 133 |
|
~% 
2415-24-9 |
| Literature: Phytochemistry (Elsevier), , vol. 21, p. 231 |
|
~% 
2415-24-9 |
| Literature: Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, , vol. 43, # 5 p. 1023 - 1025 |
|
~% 
2415-24-9 |
| Literature: Helvetica Chimica Acta, , vol. 95, # 4 p. 586 - 593 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |