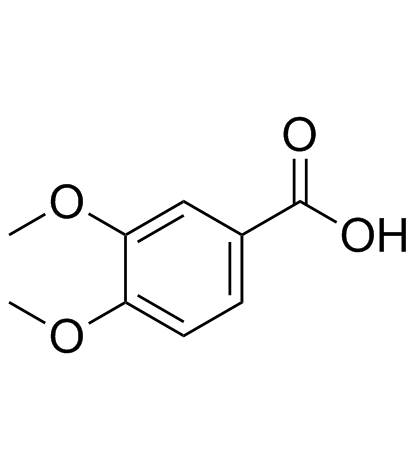
Catalpol

Catalpol structure
|
Common Name | Catalpol | ||
|---|---|---|---|---|
| CAS Number | 2415-24-9 | Molecular Weight | 362.329 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 675.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C15H22O10 | Melting Point | 203-205ºC | |
| MSDS | Chinese USA | Flash Point | 362.4±31.5 °C | |
Use of CatalpolCatalpol, an iridoid glycoside, has neuroprotective, anti-inflammatory, and anti-hepatitis virus effects.IC50 Value:Target: neuroprotective, anti-inflammatory, and anti-hepatitis virus natural product.In vitro: Catalpol could be encapsulated into composite nanofibers and induce differentiation of hASCs into neural-like cells, which might offer new avenues in nerve regeneration [1].In vivo: The pharmacokinetics of catalpol in normal and doxorubicin-induced chronic kidney disease rats after oral administration of Rehmannia glutinosa extract was determined, and the extraction recoverie of catalpol was higher than 68.24% [2]. The protective effect of catalpol on renal IRI mice through suppressing phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-endothelial nitric oxide synthase (eNOS) and against inflammation, and the possible underlying mechanism [3]. |
| Name | Catalpol |
|---|---|
| Synonym | More Synonyms |
| Description | Catalpol, an iridoid glycoside, has neuroprotective, anti-inflammatory, and anti-hepatitis virus effects.IC50 Value:Target: neuroprotective, anti-inflammatory, and anti-hepatitis virus natural product.In vitro: Catalpol could be encapsulated into composite nanofibers and induce differentiation of hASCs into neural-like cells, which might offer new avenues in nerve regeneration [1].In vivo: The pharmacokinetics of catalpol in normal and doxorubicin-induced chronic kidney disease rats after oral administration of Rehmannia glutinosa extract was determined, and the extraction recoverie of catalpol was higher than 68.24% [2]. The protective effect of catalpol on renal IRI mice through suppressing phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-endothelial nitric oxide synthase (eNOS) and against inflammation, and the possible underlying mechanism [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 675.6±55.0 °C at 760 mmHg |
| Melting Point | 203-205ºC |
| Molecular Formula | C15H22O10 |
| Molecular Weight | 362.329 |
| Flash Point | 362.4±31.5 °C |
| Exact Mass | 362.121307 |
| PSA | 161.60000 |
| LogP | -4.15 |
| Vapour Pressure | 0.0±4.7 mmHg at 25°C |
| Index of Refraction | 1.679 |
| InChIKey | LHDWRKICQLTVDL-IZSBTIMLSA-N |
| SMILES | OCC1OC(OC2OC=CC3C(O)C4OC4(CO)C23)C(O)C(O)C1O |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| RTECS | LZ5776816 |
|
~% 
Catalpol CAS#:2415-24-9 |
| Literature: Oshio,H.; Inouye,H. Phytochemistry (Elsevier), 1982 , vol. 21, p. 133 |
|
~% 
Catalpol CAS#:2415-24-9 |
| Literature: Phytochemistry (Elsevier), , vol. 21, p. 231 |
|
~% 
Catalpol CAS#:2415-24-9 |
| Literature: Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, , vol. 43, # 5 p. 1023 - 1025 |
|
~% 
Catalpol CAS#:2415-24-9 |
| Literature: Helvetica Chimica Acta, , vol. 95, # 4 p. 586 - 593 |
|
HPLC-APCI-MS/MS method for the determination of catalpol in rat plasma and cerebrospinal fluid: application to an in vivo pharmacokinetic study.
J. Pharm. Biomed. Anal. 70 , 337-43, (2012) Catalpol is a Chinese herb ingredient with potential for the treatment of neurodegenerative disorders. A high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spe... |
|
|
[Simultaneous determination and optimization of extraction process of catalpol and acteoside from rehmanniae radix].
Zhong Yao Cai 35(8) , 1318-22, (2012) To establish a method for simultaneously quantification of the Catalpol and Acteoside from Rehmanniae Radix and optimize the extraction process from Rehmanniae Radix by response surface methodology (R... |
|
|
Synergistic effects of iridoid glycosides on the survival, development and immune response of a specialist caterpillar, Junonia coenia (Nymphalidae).
J. Chem. Ecol. 38(10) , 1276-84, (2012) Plants use a diverse mix of defenses against herbivores, including multiple secondary metabolites, which may affect herbivores synergistically. Chemical defenses also can affect natural enemies of her... |
| EINECS 219-324-0 |
| catalpinoside |
| des-p-hydroxybenzoyl-catalposid |
| CATAPOL |
| 6-Hydroxy-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-2-yl hexopyranoside |
| Hexopyranoside, 1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl |
| catalposide |
| methyl iridoid glycoside |
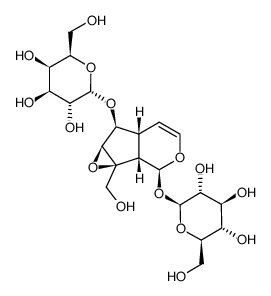
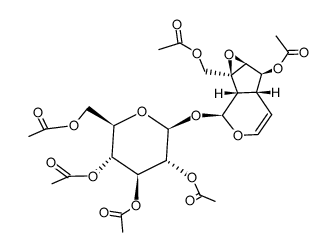
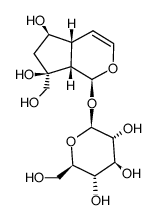
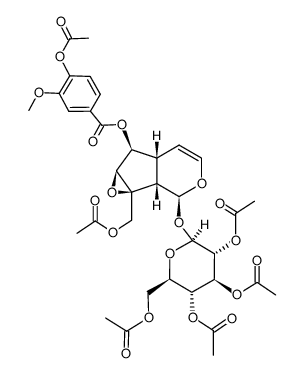
![[(1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-glucopyranosyloxy)-1b,5a,6,6a-tetrahydro-6-methoxyoxireno[4,5]cyclopenta[1,2-c]pyran-1a(2H)-yl]methyl 3,4-dimethoxybenzoate structure](https://image.chemsrc.com/caspic/423/1374308-81-2.png)