138982-67-9
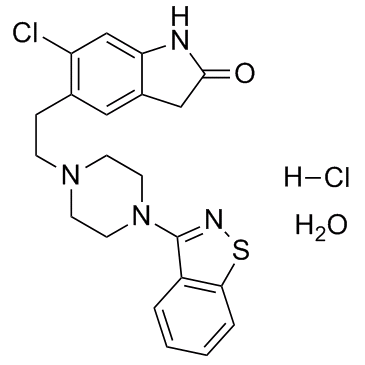
| Name | ziprasidone hydrochloride hydrate |
|---|---|
| Synonyms |
5-(2-(4-(1,2-benzisothiazol-3-yl)piperazinyl)ethyl)-6-chlorooxindole Monohydrochloride Monohydrate
cp 88059-1 5-[2-[4-(1,2-Benzisothiazol-3yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride Ziprasidone Ziprasidone Hydrochloride Monohydrate 5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-on Monohydrochloride Monohydrate 5-{2-[4-(1,2-Benzothiazol-3-yl)piperazin-1-yl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride hydrate 5-{2-[4-(1,2-Benzothiazol-3-yl)-1-piperazinyl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride hydrate Ziprasidone hydrochloride MFCD06795476 2H-Indol-2-one, 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-, hydrochloride, hydrate (1:1:1) Ziprasidone (hydrochloride monohydrate) |
| Description | Ziprasidone(CP88059) is a combined 5-HT (serotonin) and dopamine receptor antagonist which exhibits potent effects of antipsychotic activity.Target: 5-HT receptor; Dopamine receptorZiprasidone (hydrochloride) is the salt form of ziprasidone, which possesses an in vitro 5-HT2A/dopamine D2 receptor affinity ratio higher than any clinically available antipsychotic agent. In vivo, ziprasidone antagonizes 5-HT2A receptor-induced head twitch with 6-fold higher potency than for blockade of d-amphetamine-induced hyperactivity, a measure of central dopamine D2 receptor antagonism. Ziprasidone also has high affinity for the 5-HT1A, 5-HT1D and 5-HT2C receptor subtypes, which may further enhance its therapeutic potential [1]. Ziprasidone sulfoxide and sulfone were the major metabolites in human serum. The affinities of the sulfoxide and sulfone metabolites for 5-HT2 and D2 receptors are low with respect to ziprasidone, and are thus unlikely to contribute to its antipsychotic effects [2]. Ziprasidone was associated with significant differential adverse effects relative to placebo in BPM, BPD, and schizophrenia with no significant difference in weight gain in all 3 groups. Self-reported somnolence was increased across the 3 conditions. Subjects with BPM were more vulnerable to EPS than those with BPD or schizophrenia [3].Clinical indications: Bipolar I disorder; Bipolar disorder; Mania; SchizophreniaFDA Approved Date: February 2001 |
|---|---|
| Related Catalog | |
| References |
[5]. Ziprasidone |
| Boiling Point | 554.8ºC at 760 mmHg |
|---|---|
| Melting Point | 300°C |
| Molecular Formula | C21H24Cl2N4O2S |
| Molecular Weight | 467.412 |
| Exact Mass | 466.099701 |
| PSA | 85.94000 |
| LogP | 4.68760 |
| Vapour Pressure | 2.38E-12mmHg at 25°C |
| Storage condition | Room temp |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H373 |
| Precautionary Statements | P260-P280 |
| Target Organs | Central nervous system, Liver |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |