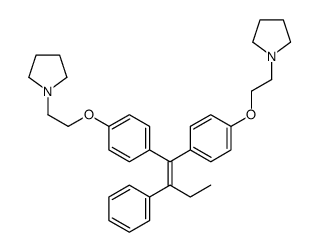
886465-70-9
| Name | 1-[2-[4-[2-phenyl-1-[4-(2-pyrrolidin-1-ylethoxy)phenyl]but-1-enyl]phenoxy]ethyl]pyrrolidine |
|---|---|
| Synonyms |
1,1 inverted exclamation marka-[(2-Phenyl-1-buten-1-ylidene)bis(4,1-phenyleneoxy-2,1-ethanediyl)]bis-pyrrolidine
Ridaifen-B |
| Description | Ridaifen-B (RID-B) is a potent antagonist of estrogen receptor α (ERα) with IC50 of 52.4 nM, a tamoxifen (HY-13757A) derivative[1]. Ridaifen-B is a high affinity, selective, inverse agonist at CB2 receptor (Ki=43.7 nM) over 17 folds CB1 receptor (Ki=732 nM). Ridaifen-B modulates G-protein (IC50=300 nM) and adenylyl cyclase activity with potency values predicted by CB2 affinity (IC50=134 nM). Ridaifen-B has anti-inflammatory, anti-cancer, and anti-osteoclastogenic effects[1][3]. |
|---|---|
| Related Catalog | |
| Target |
ERRα:52.4 nM (IC50) CB2:43.7 nM (Ki) CB1:732 nM (Ki) |
| In Vitro | Ridaifen-B (0-4 μM; 4-24 hours) damages the Jurkat cells in a dose-dependent manner within 4 hours, the IC50 rate is 4 μM. In a longer time, Ridaifen-B also injures Jurkat cells in a dose-dependent manner, similar to the previous results obtained with short-term (4-h), and the IC50 rate is about 0.4 μM[1]. Ridaifen-B (0-4 μM; 4-24 hours) induces cell apoptosis, it activates Caspase-3 in a time-dependent manner, as well as cleaved pro-Caspase-3 to active caspase-3 in ER-negative Jurkat cells[1]. Ridaifen-B (300 nM-1 μM) co-incubates with LPS significantly decreases the Emax of NO production to 19.4 μM, 22.0 μM, and 18.2 μM, respectively in RAW264.7 murine macrophages[1]. Ridaifen-B acts as an inverse agonist at CB2 receptors for modulation of G-protein activity. The potency of RID-B to modulate G-protein activation is examined by employing a non-hydrolyzable radioactive analogue of GTP, [35S]GTPγS, which hinds to G-proteins irreversibly when activated. The CB2 agonist CP-55,940 increases G-protein activation with an EC50 of 2.3 nM. However, RID-B decreases G-protein activation with an IC50 of 300 nM[2]. Consistent with modulation of G-protein activity, RID-B increases adenylyl cyclase activity in a concentration-dependent manner, elevating cAMP levels with an Imax of 398% and a potency (IC50) of 134 nM[2]. Cell Viability Assay[1] Cell Line: Jurkat cells Concentration: 0-4 μM Incubation Time: 4 hours; 24 hours Result: Damaged cell cell viability in both short- and long- term treatment. Apoptosis Analysis[1] Cell Line: Jurkat cells Concentration: 4 μM Incubation Time: 24 hours Result: Induced jurkat cells apoptosis. |
| References |
| Molecular Formula | C34H42N2O2 |
|---|---|
| Molecular Weight | 510.70900 |
| Exact Mass | 510.32500 |
| PSA | 24.94000 |
| LogP | 6.88070 |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| RIDADR | NONH for all modes of transport |