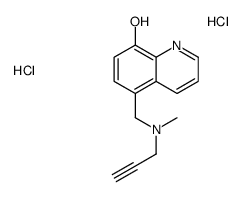
64821-19-8
| Name | 5-[[methyl(prop-2-ynyl)amino]methyl]quinolin-8-ol,dihydrochloride |
|---|---|
| Synonyms | M30 dihydrochloride |
| Description | MAO-IN-M30 dihydrochloride is an orally active, brain-permeable, and brain selective irreversible MAO-A (IC50=37 nM) and MAO-B (IC50=57 nM) inhibitor. MAO-IN-M30 dihydrochloride is a potent iron chelator and radical scavenger. MAO-IN-M30 dihydrochloride has a neuroprotective effect against Dexamethasone-induced brain cell apoptosis. MAO-IN-M30 dihydrochloride also exhibits neurorestorative activity in post MPTP and lactacystin models of Parkinson's disease[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
MAO-A:37 nM (IC50) MAO-B:57 nM (IC50) |
| In Vitro | MAO-IN-M30 (0.25 nM; 72 hours) significantly increased cell viability to ~90% after exposure to Dexamethasone[3]. MAO-IN-M30 (0-10 μM; 24 hours) enhances PC12 cell survival[4]. MAO-IN-M30 treatment significantly decreases the occurrence of fragmented DNA compared to the dexamethasone-treated group in SH-SY5Y cells[3]. Cell Viability Assay[3] Cell Line: SH-SY5Y cells Concentration: 0.25 nM Incubation Time: 72 hours Result: Significantly increased cell viability to ∼90% after exposure to Dexamethasone. Cell Viability Assay[4] Cell Line: PC12 cells Concentration: 0-10 μM Incubation Time: 24 hours Result: Enhanced the PC12 cell viability, the cell viability increasing to 85 ± 6 and 90 ± 7%. |
| In Vivo | MAO-IN-M30 (0.5-2.5 mg/kg; p.o.; once daily for 14 consecutive days) possesses neuroprotective activities[6]. Animal Model: Male C57/BL mice (20-22 g; MPTP-induced neurotoxicity in mice)[6] Dosage: 0.5, 2.5 mg/kg Administration: P.o.; once daily for 14 consecutive days Result: Significantly elevate striatal dopamine levels, reduce its metabolism, and elevate tyrosine-hydroxylase protein levels and activity. Elevated MPTP-reduced dopaminergic and transferrin receptor cell count in the SNpc. |
| References |
| Molecular Formula | C14H16Cl2N2O |
|---|---|
| Molecular Weight | 299.19600 |
| Exact Mass | 298.06400 |
| PSA | 36.36000 |
| LogP | 3.60940 |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |