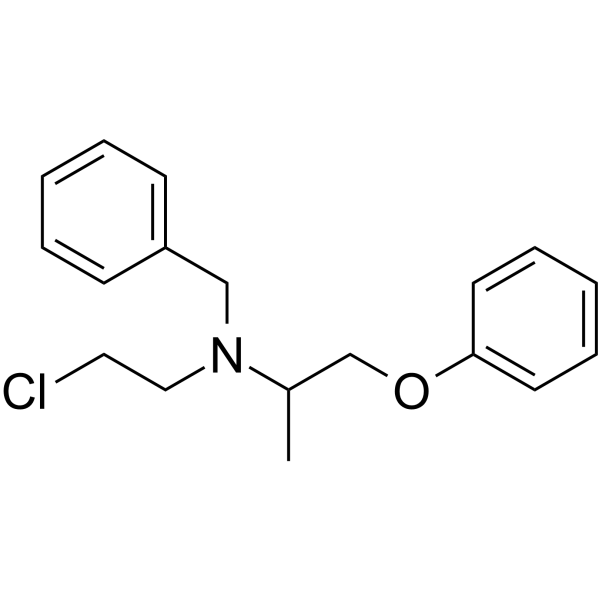
59-96-1
| Name | N-benzyl-N-(2-chloroethyl)-1-phenoxypropan-2-amine |
|---|---|
| Synonyms |
N-(2-chloroethyl)-N-(1-methyl-2-phenoxy-ethyl)benzenemethanamine
Dibenylin Dibenziran Phenoxybenzamin Dibenylene Dibenzylin Fenoxibenzamina Dibenyline Bensylyt Benzylyt phenoxybenzamine Dibenzyran |
| Description | Phenoxybenzamine is a nonselective, irreversible, orally active α-adrenoceptor antagonist that is commonly used for the research of hypertension, specifically caused by pheochromocytoma. Phenoxybenzamine also shows antitumor activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
α-adrenoceptor[1] |
| In Vitro | Phenoxybenzamine hydrochloride (0-100 μM; 96 h) markedly inhibits U251 and U87MG cells proliferation[2]. Phenoxybenzamine hydrochloride (10 μM; 24 h or 72 h) inhibits migration and invasion of U251 and U87MG cells[2]. Phenoxybenzamine hydrochloride (10 μM; 12 h) activates LINGO-1 and inhibits the TrkB-Akt pathway[2]. Phenoxybenzamine (0.1 μM-1 mM; 0-16 h) prevents hippocampal cell death after oxygen glucose deprivation[3]. Cell Proliferation Assay[2] Cell Line: U251 and U87MG cells Concentration: 0.1, 1, 10, 50 and 100 μM Incubation Time: 96 h Result: Cell proliferation was inhibited markedly, the inhibition rate being 26.5 % for U251 cells and 27.3 % for U87MG cells at 10 μM. Cell Migration Assay [2] Cell Line: U251 and U87MG cells Concentration: 10 μM Incubation Time: 24 h Result: Apparent inhibition on migration was observed, and the inhibition rate was 28.6 and 39.8 % for U251 and U87MG, respectively. Cell Invasion Assay[2] Cell Line: U251 and U87MG cells Concentration: 10 μM Incubation Time: 72 h Result: Attenuated the invasion properties of both U251 and U87MG markedly, as represented by the number of invaded cells per field declining from 365/field to 132/field (36.2 %) for U251 and 444/field to 298/field (67.1 %) for U87MG. Western Blot Analysis[2] Cell Line: U251 Concentration: 10 μM Incubation Time: 12 h Result: Decreased the protein level of TrkB, p-TrkB, and p-Akt, but Akt remained unchanged significantly. |
| In Vivo | Phenoxybenzamine hydrochloride (20 nM; s.c.; 2-day interval for 26 days) shows anti-tumorigenic effect in mice[2]. Phenoxybenzamine (1.0 mg/kg; i.v.; daily for 30 days) is neuroprotective in a rat model of severe traumatic brain injury[3]. Animal Model: Nude mice, U87MG tumor model[2] Dosage: 20 nM Administration: Subcutaneous injection, 2-day interval for 26 days Result: Reduced the tumor cells. Animal Model: Male Wistar rats (350–500 g), traumatic brain injury (TBI) model[3] Dosage: 1.0 mg/kg Administration: Intravenous injection, daily for 30 days Result: Showed significant improvements in neurological severity score (NSS) and foot fault scoring on days 14, 21, and 30. Reduced cognitive impairment associated with severe TBI and reduced the expression of pro-inflammatory genes. |
| References |
| Density | 1.102g/cm3 |
|---|---|
| Boiling Point | 381.5ºC at 760mmHg |
| Melting Point | 38-40ºC |
| Molecular Formula | C18H22ClNO |
| Molecular Weight | 303.82600 |
| Flash Point | 184.5ºC |
| Exact Mass | 303.13900 |
| PSA | 12.47000 |
| LogP | 4.19490 |
| Index of Refraction | 1.559 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2922299090 |
|---|
| HS Code | 2922299090 |
|---|---|
| Summary | 2922299090. other amino-naphthols and other amino-phenols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |