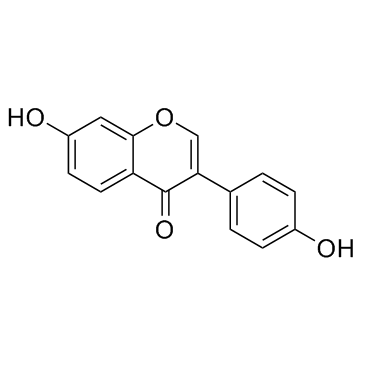
486-66-8
| Name | daidzein |
|---|---|
| Synonyms |
k251b
K 251-6 MFCD00016954 Tatoin Soybean Extract 7-Hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one (7-Hydroxy-3-(4-hydroxyphenyl)chromone 4',7-Dihydroxyisoflavone Daidzeol 7-Hydroxy-3-(4-hydroxy phenyl) chromone EINECS 207-635-4 7,4'-dihydroxyisoflavone isoaurostatin Daizeol Daidzein Daidsein |
| Description | Daidzein is a soy isoflavone, which acts as a PPAR activator. |
|---|---|
| Related Catalog | |
| Target |
PPAR-α PPAR-γ |
| In Vitro | In 3T3-L1 adipocytes, Daidzein inverses the attenuation of adiponectin gene expression by co-culture, and these effects are inhibited by the PPAR-γ specific inhibitor. Daidzein attenuates the reduction of adiponectin expression in adipocytes, and a PPAR-γ specific inhibitor abrogated this effect. Direct activation of PPAR-α and-γ by Daidzein is confirmed by a luciferase reporter assay. In HEK293T cells, Daidzein significantly increases PPAR-α transcriptional activity in a concentration-dependent manner. Although an obvious dose-dependency is not observed in PPAR-γ transcriptional activity, Daidzein also significantly increases PPAR-γ transcriptional activity over a similar range of concentrations at which Daidzein enhanced PPAR-α transcriptional activity, with a maximum increase at 25 μM[1]. Daidzein is a soy isoflavone, which upregulates the expression of Abcg1, and it promotes axonal outgrowth in cultured hippocampal neurons via estrogen receptor signaling. Daidzein is a major component of soy with structural similarity to estrogen. It exerts an anti-inflammatory effect, lowers lipid levels, and increases mitochondrial biogenesis. As an activator of nuclear receptor peroxisome proliferator-activated receptors (PPARs), Daidzein enhances transcription of PPARs-dependent genes, including liver X receptors (LXRs, Nr1h gene family in mice). Incubation with different concentrations of Daidzein, from 5 to 100 μM, increases APOE transcriptional activity[2]. |
| In Vivo | Treating Apoe KO mice with Daidzein increases Lxr and Abca1 gene expression at 1 month after stroke, showing that the absence of ApoE does not interfere with other cholesterol homeostasis genetic programs. Therefore, the findings suggest that Daidzein-induced ApoE upregulation is a critical component in fostering functional recovery in chronic stroke[2]. |
| Cell Assay | HEK293T cells are plated on 24-well plates at a cell density of approximately 2.5×104 cells/well and are grown to 70-80% confluence. Cells are then transiently transfected with a PPAR-α or PPAR-γ expression plasmid, and a plasmid containing the luciferase gene under the control of three tandem PPAR response elements (PPRE × 3 TK-luciferase) using an X-treme GENE HP DNA Transfection Reagent. Renilla luciferase control vectors are co-transfected to control for transfection efficiency. After transfection, cells are cultured for another 24 h in medium containing DMSO or various concentrations (6.25, 12.5, 25 μM) of Daidzein. Cells are lysed, and luciferase activity is measured and expressed as fold induction, that is normalized to the activity of the renilla luciferase control plasmid[1]. |
| Animal Admin | Mice[2] Experiments are performed in 10- to 11-week-old male C57 (C57 bl/6) and Apoe KO (C57 background) mice. For long-term stroke recovery, mice receive Moxifloxacin (100 mg/kg) for 3 d. The prophylactic antibiotic treatment is shown to effectively reduce mortality in an animal model of stroke by attenuating peripheral infection. In addition, saline is subcutaneously administered daily, and hydrogel (Clear H2O) is given to prevent dehydration. With the implementation of poststroke care (antibiotic regimen, rehydration, and feeding hydrogels with soft diet) during the acute period (<1 week), mice start to regain their body weight by day 5 and continue to recover from stroke. Animals are randomly selected for vehicle or Daidzein treatment. Vehicle or Daidzein (10 mg/kg) is administered subcutaneously within 30 min of reperfusion after confirming the reperfusion of blood flow, daily for 7 d and then every other day up to 1 month. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 512.8±50.0 °C at 760 mmHg |
| Melting Point | 315-323°C (dec.) |
| Molecular Formula | C15H10O4 |
| Molecular Weight | 254.238 |
| Flash Point | 201.2±23.6 °C |
| Exact Mass | 254.057907 |
| PSA | 70.67000 |
| LogP | 2.78 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.699 |
| Storage condition | 2-8°C |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P280-P305 + P351 + P338-P337 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/38 |
| Safety Phrases | S24-S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DJ3100040 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |




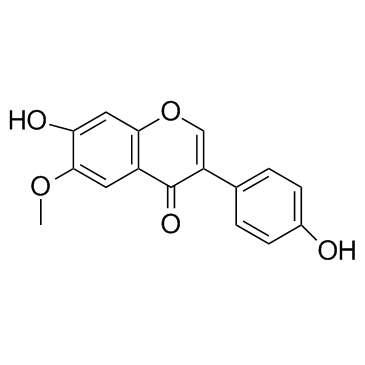
![[4-(7-hydroxy-4-oxochromen-3-yl)phenyl] hexanoate structure](https://image.chemsrc.com/caspic/011/602329-51-1.png)