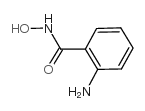
28230-32-2
| Name | 3-hydroxy-1,2,3-benzotriazin-4-one |
|---|---|
| Synonyms |
HOOBT
EINECS 248-916-1 3-hydroxybenzo[d]1,2,3-triazin-4-one 3-hydroxy-3H-benzo[d][1,2,3]triazin-4-one DHBT oxohydroxybenzotriazole 4-oxo-3,4-dihydro-3-hydroxy-1,2,3-benzotriazine 3-Hydroxy-1,2,3-benzotriazin-4(3H)-one 3-Hydroxy-4-ketobenzotriazine 3-Hydroxy-1,2,3-ben zotriazin-4(3H)-one 3,4-Dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine 1,2,3-Benzotriazin-4(3H)-one,3-hydroxy MFCD00042803 3,4-dihydro-3-hydroxy-4-keto-1,2,3-benzotriazine 3-Hydroxy-3,4-dihydro-4-oxo-1,2,3-benzotriazine |
| Description | HODHBt (HOOBt) inhibits STAT5-SUMO interaction by blocking SUMOylation of phosphorylated STAT5. HODHBt enhances the magnitude of IL-15 signaling and significantly increases the natural killer (NK) cell cytotoxicity phenotype and function and the generation of cytokine-induced memory-like (CIML) natural killer (NK) cells. HODHBt can be used for research of HIV-infection and cancer[1]. |
|---|---|
| Related Catalog | |
| Target |
STAT5 |
| In Vitro | HODHBt (100 μM; 24 h, and 48 h respectively) increases IL-15-mediated STAT phosphorylation in NK cells and enhances the cytotoxic profile of NK cells[1]. HODHBt (100 μM; 24 h) enhances IL-15-mediated (100 ng/mL) cytotoxicity of NK cells against HIV-infected cells (K562 cells)[1]. HODHBt (100 μM; 24 h) enhances IL-15-mediated (100 ng/mL, 6 h) cytotoxicity of NK cells against cancer cell lines (A2780 and U877; OCILy1 and OCILy10)[1]. HODHBt (100 μM; 7 d) results in enhanced memory response upon recall in generation of human CIML NK cells in vitro[1]. |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.7±25.0 °C at 760 mmHg |
| Melting Point | 184-189°C |
| Molecular Formula | C7H5N3O2 |
| Molecular Weight | 163.133 |
| Flash Point | 164.7±23.2 °C |
| Exact Mass | 163.038177 |
| PSA | 68.01000 |
| LogP | 0.43 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.739 |
| Storage condition | 2-8°C |
| Symbol |



GHS02, GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H226-H312 + H332-H315-H319-H335-H360D |
| Precautionary Statements | P201-P261-P280-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S53-S26-S35-S36/37-S45-S37/39 |
| RIDADR | UN 3379 3/PG 1 |
| WGK Germany | 3 |
| Hazard Class | 3.0 |
| HS Code | 2933699090 |
|
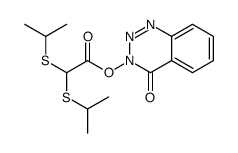
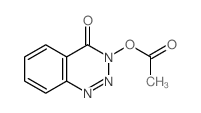
~77% 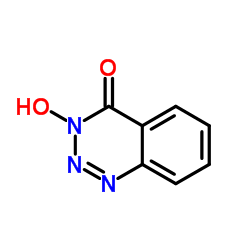
28230-32-2 |
| Literature: FRUTAROM LTD. Patent: WO2005/7634 A1, 2005 ; Location in patent: Page 39 ; |
|
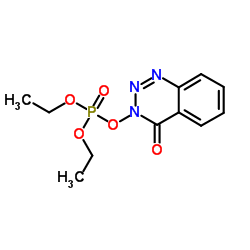
~% 
28230-32-2 |
| Literature: Jakobsen, Mogens Havsteen; Buchardt, Ole; Holm, Arne; Meldal, Morten Synthesis, 1990 , # 11 p. 1008 - 1010 |
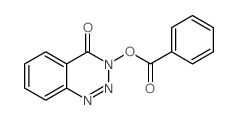
|
~% 
28230-32-2 |
| Literature: Vaughan, Keith; Wilman, Derry E. V.; Wheelhouse, Richard T.; Stevens, Malcolm F. G. Magnetic Resonance in Chemistry, 2002 , vol. 40, # 4 p. 300 - 302 |
| Precursor 3 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |