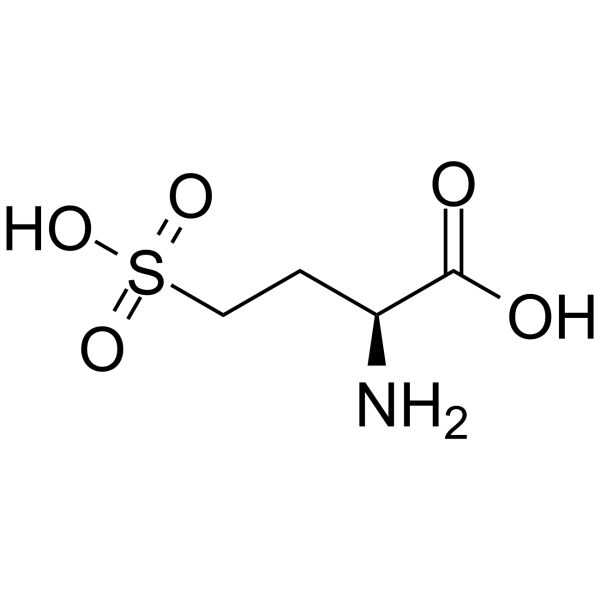
14857-77-3
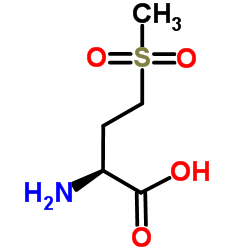
| Name | L-Homocysteic acid |
|---|---|
| Synonyms |
L-2-Amino-4-sulfobutyric acid
(2S)-2-amino-4-sulfobutanoic acid |
| Description | L-Homocysteic acid (L-HCA) is an endogenous excitatory amino acid that acts as a NMDA receptor agonist (EC50: 14 μM). L-Homocysteic acid is neurotoxic, and can be used in the research of neurological disorders[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
NMDA Receptor:14 μM (EC50) |
| In Vitro | L-Homocysteic acid activates NMDA receptor with an EC50 value of 14 μM[1]. L-Homocysteic acid (100 μM) induces large currents (1.8 nA) that is insensitive to the NMDA receptor-antagonist mixture in Purkinje cells[1]. L-Homocysteic acid (250 μM, 30 min) potently induces an acute excitotoxic reaction in ex vivo chick embryo retina[2]. L-Homocysteic acid (0-2 mM, 48 h) induces a concentration-dependent neurotoxic effect in rat primary neurons[3]. |
| In Vivo | L-Homocysteic acid (intraperitoneal injection, 4-11 mmol/kg) elicits seizures in rats during early postnatal development[4]. L-Homocysteic acid (intraperitoneal injection, 100-1500 mg/kg) partially substitutes for NMDA, producing maximum values of 61-67% NMDA-lever responding at doses of 1000 and 560 mg/kg, respectively in Sprague-Dawley rats[5]. Animal Model: Male albino rats of the Wistar strain[4] Dosage: 4, 5.5, 8, 11 mM/kg Administration: Intraperitoneal injection, daily for 14 days Result: Induced flexion seizures at 4 mmol/kg. Led to intense tail flicking, pivoting, and locomotion. Decreased ECoG (electrocorticograms) activity for 5-9 min. |
| References |
[4]. P Mares, et al. Convulsant action of D,L-homocysteic acid and its stereoisomers in immature rats. |
| Density | 1.638g/cm3 |
|---|---|
| Molecular Formula | C4H9NO5S |
| Molecular Weight | 183.18300 |
| Exact Mass | 183.02000 |
| PSA | 126.07000 |
| LogP | 0.45730 |
| Index of Refraction | 1.56 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2922499990 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu Tetrahedron Letters, 1998 , vol. 39, # 9 p. 1009 - 1012 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu Tetrahedron Letters, 1998 , vol. 39, # 9 p. 1009 - 1012 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu Tetrahedron Letters, 1998 , vol. 39, # 9 p. 1009 - 1012 |
|
~% 
14857-77-3 |
| Literature: Watkins,J.C. Journal of Medicinal and Pharmaceutical Chemistry, 1962 , vol. 5, p. 1187 - 1199 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu Tetrahedron Letters, 1998 , vol. 39, # 9 p. 1009 - 1012 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu Tetrahedron Letters, 1998 , vol. 39, # 9 p. 1009 - 1012 |
|
~%
Detail
|
| Literature: Nomoto, Shinya; Shimoyama, Akira; Shiraishi, Susumu; Seno, Tomoyuki; Sahara, Denzo Bioscience, Biotechnology and Biochemistry, 1998 , vol. 62, # 4 p. 643 - 649 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |