215030-90-3
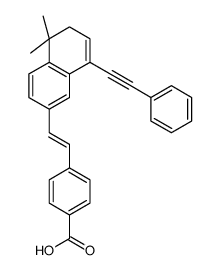
| Name | 4-{(E)-2-[5,5-Dimethyl-8-(phenylethynyl)-5,6-dihydro-2-naphthalen yl]vinyl}benzoic acid |
|---|---|
| Synonyms |
4-[(E)-(5 6-Dihydro-5,5-dimethyl-8-phenylethynyl-2-naphthalenyl)ethenyl]benzoic acid
(E)-4-[2-[2-[[(p-methoxyphenyl)sulfonyl]amino]phenyl]ethenyl]pyridine (E)-4-(2-(2-(N-(4-METHOXYBENZENESULFONYL)AMINO)PHENYL)VINYL)PYRIDINE (E)-4-[2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)naphthalen-2-yl]ethen-1-yl]benzoic acid Benzenesulfonamide,4-methoxy-N-[2-[2-(4-pyridinyl)ethenyl]phenyl]-,(E) (E)-4-[2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)naphthalene-2-yl]ethen-1-yl]benzoic acid Benzenesulfonamide,4-methoxy-N-[2-[(1E)-2-(4-pyridinyl)ethenyl]phenyl] (E)-4-[2-{2-[N-(4-methoxybenzenesulfonyl)amino]phenyl}ethenyl ]pyridine (E)-4-[2-[2-[N-[(p-Methoxyphenyl)sulfonyl]amino]phenyl]ethenyl]pyridine (E)-4-[2-[2-(p-methoxy-benzene-sulfonamide)phenyl]ethenyl]pyridine BMS-204493 |
| Description | BMS493 is an inverse pan-retinoic acid receptor (RAR) agonist. BMS493 increases nuclear corepressor interaction with RARs. BMS493 also could prevent retinoic acid-induced differentiation[1][2]. |
|---|---|
| Related Catalog | |
| Target |
RAR |
| In Vitro | BMS493 (100 nM; 6 days; ALDHhi UCB cells) treatment shows a twofold increase in the number of ALDHhi cells available for transplantation compared with untreated controls. Newly expanded ALDHhi cells shows increased numbers of CD34 and CD133-positive cells, as well as a reduction in CD38 expression, a marker of hematopoietic cell differentiation[1]. Cell Viability Assay[1] Cell Line: ALDHhi UCB cells Concentration: 100 nM Incubation Time: 6 days Result: Showed a twofold increase in the number of ALDHhi cells available for transplantation compared with untreated controls. |
| In Vivo | Intrapancreatic transplantation of cell progeny after expansion of ALDHhi cells with or without BMS493 shows no reduction of hyperglycemia in Streptozotocin-treated NOD/SCID mice. Thus, Umbilical cord blood (UCB)-derived ALDHhi cells effectively lost islet regenerative capacity during ex vivo expansion[1]. |
| References |
| Molecular Formula | C29H24O2 |
|---|---|
| Molecular Weight | 404.50000 |
| Exact Mass | 404.17800 |
| PSA | 37.30000 |
| LogP | 6.67160 |
| Hazard Statements | H413 |
|---|---|
| RIDADR | NONH for all modes of transport |