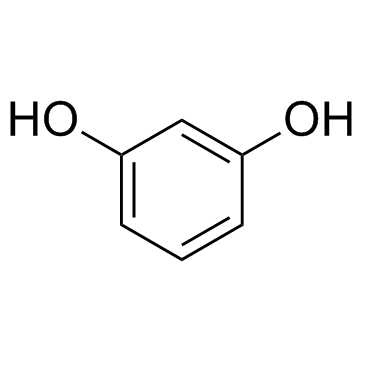
596-09-8
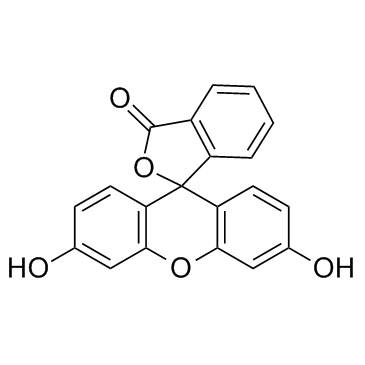
| Name | Fluorescein Diacetate |
|---|---|
| Synonyms |
EINECS 209-877-6
3',6'-Bis(acetyloxy)spiro(isobenzofuran-1(3H),9'-(9H)xanthen)-3-one 3-Oxospiro(isobenzofuran-1(3H),9'-(9H)xanthene)-3',6'-diyl diacetate MFCD00005062 3-Oxo-3H-spiro[2-benzofuran-1,9'-xanthene]-3',6'-diyl diacetate (6'-acetyloxy-3-oxospiro[2-benzofuran-1,9'-xanthene]-3'-yl) acetate Fluorescein Diacetate |
| Description | Fluorescein diacetate is a cell permeable esterase-substrate. Fluorescein diacetate can be used as a fluorogenic substrate for hGSTP1-1. |
|---|---|
| Related Catalog | |
| In Vitro | Fluorescein diacetate (FDA) is an acetylated derivative of the green fluorescent dye fluorescein[1]. Fluorescein diacetate (FDA), a fluorescent probe used for vital staining, is a fluorescently activated by esterolytic activity of human Pi-class glutathione S-transferase (hGSTP1) selectively among various cytosolic GSTs. Fluorescence activation of Fluorescein diacetate susceptible to GST inhibitors is observed in MCF7 cells exogenously overexpressing hGSTP1, but not in cells overexpressing hGSTA1 or hGSTM1. Fluorescein diacetate can be used as a fluorogenic substrate for hGSTP1-1. To investigate whether the fluorescence activation is due to hGSTP1 activity, Fluorescein diacetate is incubated with recombinant hGSTP1-1 and GSH in vitro. Remarkable fluorescence activation is observed in the presence of both hGSTP1-1 and GSH, whereas only slight activation is observed in the absence of either of them or when the enzyme is heat inactivated. This suggests that the fluorescence activation of Fluorescein diacetate depends on hGSTP1-1 activity. From the linear relationship between the rate of increase in fluorescence and the hGSTP1-1 concentration, the specific activity of the enzyme for 1 μM Fluorescein diacetate is determined to be 79±15 nmol/min/mg protein. Fluorescein diacetate is applicable as a fluorogenic substrate for evaluating inhibitors of GSTP1-1 in vitro. For Fluorescein diacetate as a substrate, both Ethacrynic acid (EA) and NBDHEX suppress the hGSTP1-1-dependent fluorescent increase in a concentration-dependent manner, with IC50s of 3.3±0.5 μM and 0.61±0.04 μM, respectively[2]. |
| Cell Assay | MCF7 cells (2-3×105) are seeded in a 35-mm glass bottom dish before the experiment. Prior to imaging, cells are washed with 1 mL PBS, then incubated in 1 mL Hanks’ Balanced Salt solution (HBSS(+) without phenol red) containing 1 μM Fluorescein diacetate (0.1% DMSO as a cosolvent) for 5 min at 37°C. Cells are washed twice with 1 mL PBS and 1 mL HBSS is added before imaging. Fluorescence images of fluorescein and DsRed-Express2 were acquired in the FITC channel (excitation at 473 nm) and the DsRed channel (excitation 559 nm). The 16-bit images obtained are analysed[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 604.7±55.0 °C at 760 mmHg |
| Melting Point | 200-203 °C(lit.) |
| Molecular Formula | C24H16O7 |
| Molecular Weight | 416.380 |
| Flash Point | 264.0±31.5 °C |
| Exact Mass | 416.089600 |
| PSA | 88.13000 |
| LogP | 2.77 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.681 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~72% 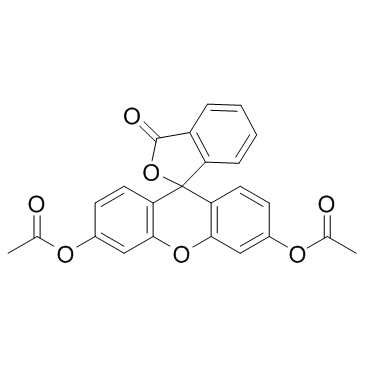
596-09-8 |
| Literature: WO2008/73764 A2, ; Page/Page column 23 ; |
|
~% 
596-09-8 |
| Literature: Journal of the Association of Official Agricultural Chemists, , vol. 34, p. 114,115 |
|
~% 
596-09-8 |
| Literature: Tetrahedron Letters, , vol. 44, # 52 p. 9359 - 9362 |
|
~% 
596-09-8 |
| Literature: Tetrahedron Letters, , vol. 44, # 52 p. 9359 - 9362 |
|
~% 
596-09-8 |
| Literature: Journal of the American Chemical Society, , vol. 30, p. 862 |
|
~% 
596-09-8 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 183, p. 31 Justus Liebigs Annalen der Chemie, , vol. 372, p. 109 |
|
~93% 
596-09-8 |
| Literature: Dyes and Pigments, , vol. 89, # 2 p. 120 - 126 |
|
~% 
596-09-8 |
| Literature: Chemische Berichte, , vol. 7, p. 1212 Justus Liebigs Annalen der Chemie, , vol. 183, p. 31 Justus Liebigs Annalen der Chemie, , vol. 372, p. 109 |
|
~% 
596-09-8 |
| Literature: Chemische Berichte, , vol. 7, p. 1212 Justus Liebigs Annalen der Chemie, , vol. 183, p. 31 Justus Liebigs Annalen der Chemie, , vol. 372, p. 109 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |