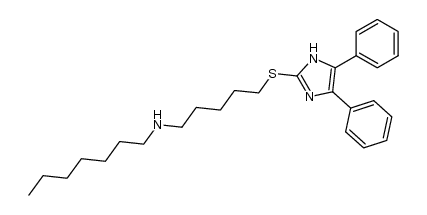
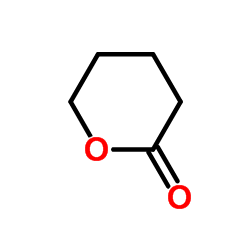
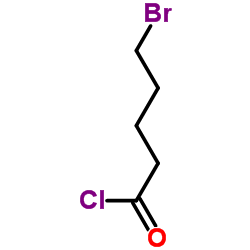
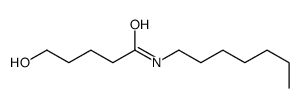
130804-35-2
| Name | 3-(2,4-difluorophenyl)-1-[5-[(4,5-diphenyl-1H-imidazol-2-yl)sulfanyl]pentyl]-1-heptylurea |
|---|---|
| Synonyms |
N'-(2,4-difluorophenyl)-N-[5-(4,5-diphenyl-1H-imidazol-2-ylthio)pentyl]-N-heptylurea
urea,n'-(2,4-difluorophenyl)-n-[5-[(4,5-diphenyl-1h-imidazol-2-yl)thio]pentyl]-n-heptyl Lecimibide Lecimibide (USAN) |
| Description | Lecimibide (DuP 128) is a potent and specific acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor for antihyperlipidemia research[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Lecimibide (DuP 128)(10 μM, 24 h)可以抑制 HepG2 细胞 85% 的细胞酯化反应[1]。 |
| In Vivo | Lecimibide (DuP 128)(i.v., 2.2 mg/kg/day) 在饲养高脂肪和胆固醇的猪中,显著降低了总血浆甘油三酯和极低密度脂蛋白(VLDL)甘油三酯浓度,分别降低了 36% 和 31%。而对总胆固醇、VLDL 胆固醇、LDL 胆固醇、HDL 胆固醇或 LDL apoB 浓度没有显著影响[2]。 |
| References |
| Density | 1.21g/cm3 |
|---|---|
| Molecular Formula | C34H40F2N4OS |
| Molecular Weight | 590.76900 |
| Exact Mass | 590.28900 |
| PSA | 89.81000 |
| LogP | 9.80240 |
| Index of Refraction | 1.613 |
|
~58% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
|
~% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
|
~% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
|
~% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
|
~% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
|
~% 
130804-35-2 |
| Literature: Higley; Wilde; Maduskuie; Johnson; Pennev; Billheimer; Robinson; Gillies; Wexler Journal of Medicinal Chemistry, 1994 , vol. 37, # 21 p. 3511 - 3522 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |