1024033-43-9
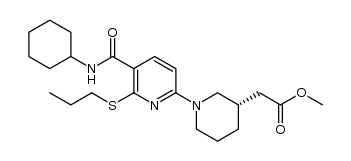
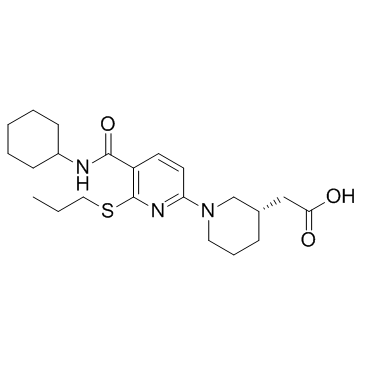
| Name | 2-[(3S)-1-[5-(cyclohexylcarbamoyl)-6-propylsulfanylpyridin-2-yl]piperidin-3-yl]acetic acid |
|---|---|
| Synonyms |
{(3s)-1-[5-(Cyclohexylcarbamoyl)-6-(Propylsulfanyl)pyridin-2-Yl]piperidin-3-Yl}acetic Acid
2-[(3S)-1-[5-(cyclohexylcarbamoyl)-6-propylsulfanyl-pyridin-2-yl]-3-piperidyl]acetic acid 14M AZD-4017 |
| Description | AZD 4017 is a potent, selective 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) inhibitor, with an IC50 of 7 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 7 nM (11β-HSD1)[1]. |
| In Vitro | AZD 4017 displays excellent selectivity versus the related enzymes 11-βHSD2, 17β-HSD1, 17β-HSD3 (all IC50>30 μM) and shows no measurable activity against the glucocorticoid and mineralocorticoid receptors. Despite having high potency for the human form of 11β-HSD1, AZD 4017 shows much reduced activity across species with the exception of cynomolgous monkey (IC50=0.029 μM). Additionally, as it is believed that adipose is a key target organ, inhibition of 11β-HSD1 activity is measured in isolated human adipocytes from nondiabetic volunteers. AZD 4017 is shown to be a potent inhibitor in this key target tissue (IC50=0.002 μM) in good agreement with the enzyme potency, thus providing some confidence that AZD 4017 is not restricted from adipose tissue by the fact that it was acidic[1]. |
| In Vivo | Since AZD 4017 has lower potency against the mouse enzyme, only a limited number of preclinical pharmacodynamic measurements are performed. Increasing the dose further led to a maximal effect of approximately 70% inhibition at 1500 mg/kg, equivalent to 10×IC50 in the mouse, demonstrating the dose dependent inhibition of 11β-HSD1 by AZD 4017 in this model[1]. |
| References |
| Molecular Formula | C22H33N3O3S |
|---|---|
| Molecular Weight | 419.58100 |
| Exact Mass | 419.22400 |
| PSA | 107.83000 |
| LogP | 4.79320 |
|
~% 
1024033-43-9 |
| Literature: Scott, James S.; Bowker, Suzanne S.; Deschoolmeester, Joanne; Gerhardt, Stefan; Hargreaves, David; Kilgour, Elaine; Lloyd, Adele; Mayers, Rachel M.; McCoull, William; Newcombe, Nicholas J.; Ogg, Derek; Packer, Martin J.; Rees, Amanda; Revill, John; Schofield, Paul; Selmi, Nidhal; Swales, John G.; Whittamore, Paul R. O. Journal of Medicinal Chemistry, 2012 , vol. 55, # 12 p. 5951 - 5964 |
|
~% 
1024033-43-9 |
| Literature: Scott, James S.; Bowker, Suzanne S.; Deschoolmeester, Joanne; Gerhardt, Stefan; Hargreaves, David; Kilgour, Elaine; Lloyd, Adele; Mayers, Rachel M.; McCoull, William; Newcombe, Nicholas J.; Ogg, Derek; Packer, Martin J.; Rees, Amanda; Revill, John; Schofield, Paul; Selmi, Nidhal; Swales, John G.; Whittamore, Paul R. O. Journal of Medicinal Chemistry, 2012 , vol. 55, # 12 p. 5951 - 5964 |
|
~% 
1024033-43-9 |
| Literature: Scott, James S.; Bowker, Suzanne S.; Deschoolmeester, Joanne; Gerhardt, Stefan; Hargreaves, David; Kilgour, Elaine; Lloyd, Adele; Mayers, Rachel M.; McCoull, William; Newcombe, Nicholas J.; Ogg, Derek; Packer, Martin J.; Rees, Amanda; Revill, John; Schofield, Paul; Selmi, Nidhal; Swales, John G.; Whittamore, Paul R. O. Journal of Medicinal Chemistry, 2012 , vol. 55, # 12 p. 5951 - 5964 |
|
~% 
1024033-43-9 |
| Literature: Scott, James S.; Bowker, Suzanne S.; Deschoolmeester, Joanne; Gerhardt, Stefan; Hargreaves, David; Kilgour, Elaine; Lloyd, Adele; Mayers, Rachel M.; McCoull, William; Newcombe, Nicholas J.; Ogg, Derek; Packer, Martin J.; Rees, Amanda; Revill, John; Schofield, Paul; Selmi, Nidhal; Swales, John G.; Whittamore, Paul R. O. Journal of Medicinal Chemistry, 2012 , vol. 55, # 12 p. 5951 - 5964 |
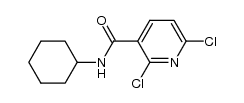
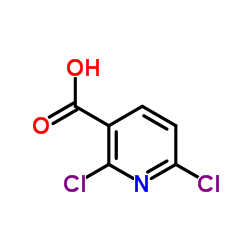
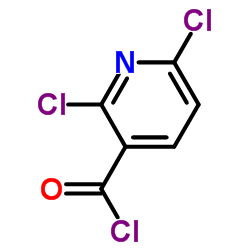
| Precursor 4 | |
|---|---|
| DownStream 0 | |