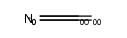
1226056-71-8
| Name | N-Benzyl-2-(pyrimidin-4-ylamino)thiazole-4-carboxamide |
|---|---|
| Synonyms |
Thiazovivin
N-Benzyl-2-(pyrimidin-4-ylamino)-1,3-thiazole-4-carboxamide N-Benzyl-2-(4-pyrimidinylamino)-1,3-thiazole-4-carboxamide |
| Description | Thiazovivin is a potent ROCK inhibitor, which can protect human embryonic stem cells. |
|---|---|
| Related Catalog | |
| Target |
ROCK |
| In Vitro | Thiazovivin is a ROCK inhibitor. Thiazovivin (2 μM) inhibits ROCK activity and protects human embryonic stem cells (hESCs). Thiazovivin significantly increases the survival of hESCs after dissociation while maintaining pluripotency. Thiazovivin enhances cell-ECM adhesion-mediated integrin signaling. Thiazovivin also stabilizes E-cadherin after cell dissociation to protect hESCs from death under ECM-free conditions[1]. Thiazovivin increases cellular attachment of embryo-derived stem-like cells (eSLCs) of cattle and formation of primary colonies on the feeder layer. Thiazovivin reinforces putative colony outgrowth and supports the expansion of eSLC cultures during the subculture for passaging. Furthermore, Thiazovivin causes greater expression of ectodermal lineage-specific genes in eSLCs of cattle[2]. |
| Cell Assay | For cell proliferation assays, embryo-derived stem-like cells (eSLCs) are newly passaged and cultured in 3i system for 48 h on the feeder-free condition to prevent contamination of the BrdU-positive feeder cells. Cells are fixed with 4% paraformaldehyde in PBS (pH 7.4) at 37°C for 2 h, acid-treated with 2 N HCl in PBS for 30 min at 45°C, equilibrated with 0.1 M borate buffer (pH 8.5), and finally incubated with blocking buffer (20% Calf serum; 0.1% Triton X-100; 1% DMSO in PBS) for 2 h. Fixed cells are immunostained with antibodies against anti-BrdU mouse monoclonal antibody IgG followed by incubation with the secondary antibodies FITC conjugated goat anti-mouse IgG. The treated cells are covered with slow-fade anti-fade with DAPI for nuclear staining and covered with a glass coverslip. Images are captured with the fluorescence microscope[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C15H13N5OS |
| Molecular Weight | 311.362 |
| Exact Mass | 311.084076 |
| PSA | 108.04000 |
| LogP | 2.00 |
| Appearance | , white to beige to brown |
| Index of Refraction | 1.691 |
| Storage condition | Store at -20°C |
| Water Solubility | DMSO: soluble20mg/mL, clear |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934100090 |
|
~54% 
1226056-71-8 |
| Literature: Ries, Oliver; Granitzka, Markus; Stalke, Dietmar; Ducho, Christian Synthetic Communications, 2013 , vol. 43, # 21 p. 2876 - 2882 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |