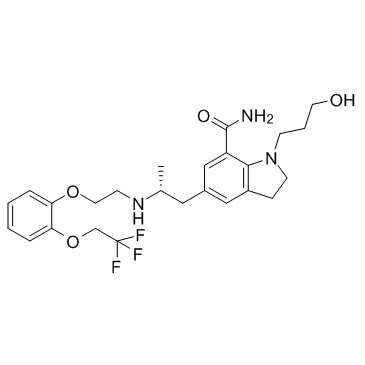
160970-54-7
| Name | 1-(3-hydroxypropyl)-5-[(2R)-2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-2,3-dihydroindole-7-carboxamide |
|---|---|
| Synonyms |
1-(3-Hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-7-indolinecarboxamide
KAD 3213 1-(3-hydroxypropyl)-5-[(2R)-2-({2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}amino)propyl]-2,3-dihydro-1H-indole-7-carboxamide [3H]-Silodosin Rapaflo [14C]-Silodosin UNII-CUZ39LUY82 Silodyx Urief Silodosin KMD-3213 |
| Description | Silodosin (Rapaflo; KMD-3213) is an α1-adrenoceptor antagonist with high uroselectivity; In treatment of dysuria.IC50 Value:Target: Adrenergic Receptorin vitro: Silodosin potently inhibited 2-[2-(4-hydroxy-3-[125I]iodophenyl)ethylaminomethyl]-alpha-tetralone binding to the cloned human alpha 1a-AR, with a Ki value of 0.036 nM, but had 583- and 56-fold lower potency at the alpha 1b- and alpha 1d-ARs, respectively. Silodosin inhibited norepinephrine-induced increases in intracellular Ca2+ concentrations in alpha 1a-AR-expressing Chinese hamster ovary cells with an IC50 of 0.32 nM but had a much weaker inhibitory effect on the alpha 1b- and alpha 1d-ARs.in vivo: Using pharmacologically well characterized native rat tissues [submaxillary gland (alpha 1A-AR-expressing tissue), liver (alpha 1B-AR-expressing tissue), and heart (mixed alpha 1A- and alpha 1B-AR-expressing tissue)], binding studies showed that inhibition curves for Silodosin in submaxillary gland and liver best fit a one-site model (with Ki values of 0.15 and 16 nM, respectively), whereas Silodosin had high and low affinity sites in heart membranes. Chloroethylclonidine treatment of rat heart membranes completely eliminated the low affinity sites for Silodosin. Furthermore, in human liver and prostate Silodosin could identify high and low affinity sites, the Ki values of which corresponded well to those for the cloned human alpha 1a- and alpha 1b-ARs, respectively. Moreover, the affinity of Silodosin was found to be approximately 10-fold higher at the cloned human alpha 1a-AR than at the cloned rat alpha 1a-AR.v |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 601.4±55.0 °C at 760 mmHg |
| Melting Point | 107 °C |
| Molecular Formula | C25H32F3N3O4 |
| Molecular Weight | 495.534 |
| Flash Point | 317.5±31.5 °C |
| Exact Mass | 495.234497 |
| PSA | 97.05000 |
| LogP | 2.52 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Hazard Codes | Xi |
|---|