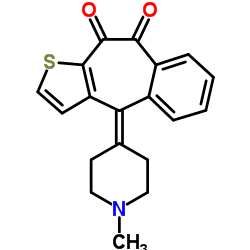
34580-13-7
| Name | 10-(1-methylpiperidin-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one |
|---|---|
| Synonyms |
Ketotifeno
Ketotiphene Ketotifene Ketotiphen Ketotifenum ketotifen Zaditen |
| Description | Ketotifen (HC 20-511) is an orally active second-generation noncompetitive histamine 1 (H1) receptor blocker and mast cell stabilizer. Ketotifen can block 6-phosphogluconate dehydrogenase (PGD) in vitro. Ketotifen also has antiviral activity against SARS-CoV-2 and Influenza virus. Ketotifen can be used to the research of autoimmune encephalomyelitis (EAE) and asthma attack prevention[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Histamine 1, SARS-CoV-2, Influenza virus[1][3][4] |
| In Vitro | Ketotifen (0-100 μM; 2 or 4 days) inhibits SARS-CoV-2 with an EC50 of 48.9 μM; and increases the percentage inhibition of SARS-CoV-2 to 79%, 83% and 93% when co-administers with 25, 50 and 100 μM Indomethacin, respectively[3]. Ketotifen (0-50 μM; 24 h) has inhibitory activity against PR8, pH1N1 and H3N2 with EC50s of 5.9 μM, 33.7 μM and 48.5 μM, respectively; and exhibits relatively low cytotoxicity in MDCK cells (EC50=291 μM)[4]. |
| In Vivo | Ketotifen (80 mg/kg; i.g.; daily for 3 days) reduces end organ damage and mortality in mice infected with influenza virus[4]. Ketotifen (0.4 mg/kg; i.p.; daily for 10 days) reduces encephalomyelitis (EAE) prevalence and severity[5]. Animal Model: Female C57BL/6 mice (4-6 weeks; intranasal infection with 1×103 TCID50 of PR8 in 30 μL of DMEM)[4] Dosage: 80 mg/kg Administration: i.g.; daily for 3 days Result: Reduced end organ damage and mortality in infected mice. Animal Model: Female C57BL/6 mice (5-6 weeks old; subcutaneously immunized with 150 μg of MOG35-55 peptide containing 4 mg/mL of Mycobacterium tuberculosis)[5] Dosage: 0.4 mg/kg Administration: i.p.; daily for 10 days (from the 7th day of infection) Result: Reduced EAE prevalence and severity; reduced oxidative stress status and inflammasome activation at the CNS; reduced the amount of T cells, especially Th1, in the CNS; downregulated local mRNA expression for mast cell enzymes and preserves blood-CNS barrier permeability; triggered lymphocyte accumulation in draining lymph nodes. |
| References |
| Density | 1.236g/cm3 |
|---|---|
| Boiling Point | 488.9ºC at 760mmHg |
| Melting Point | 152-153ºC |
| Molecular Formula | C19H19NOS |
| Molecular Weight | 309.42500 |
| Flash Point | 249.5ºC |
| Exact Mass | 309.11900 |
| PSA | 48.55000 |
| LogP | 3.95230 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|
| Precursor 9 | |
|---|---|
| DownStream 3 | |
![4H-Benzo[4,5]cyclohepta[1,2-b]thiophene-4-ol,10-methoxy-4-(1-methyl-4-piperidinyl) structure](https://image.chemsrc.com/caspic/313/59743-88-3.png)
![10-hydroxy-10-(1-methylpiperidin-4-yl)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one structure](https://image.chemsrc.com/caspic/142/126939-27-3.png)
![10-Methoxy-4H-benzo[4,5]cyclohepta[1,2-b]thiophen-4-one structure](https://image.chemsrc.com/caspic/475/59743-84-9.png)
![4-(10-bromo-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ylidene)-1-methyl-piperidine structure](https://image.chemsrc.com/caspic/347/34580-12-6.png)
![4H-Benzo[4,5]cyclohepta[1,2-b]thiophen-4-one,9,10-dihydro structure](https://image.chemsrc.com/caspic/200/1622-55-5.png)
![10-bromo-4H-benzo[4,5]cyclohepta[1,2-b]thiophene-4-one structure](https://image.chemsrc.com/caspic/385/34580-11-5.png)
![10-(1-methyl-1-oxidopiperidin-1-ium-4-ylidene)-5H-benzo[1,2]cyclohepta[3,4-b]thiophen-4-one structure](https://image.chemsrc.com/caspic/210/88456-70-6.png)
![10-(1-methylpiperidin-4-ylidene)-4,5-dihydrobenzo[1,2]cyclohepta[3,4-b]thiophen-4-ol structure](https://image.chemsrc.com/caspic/447/43076-12-6.png)