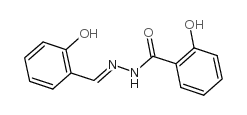
3232-36-8
| Name | SCS,Salicylidenesalicylhydrazide |
|---|---|
| Synonyms |
2-hydroxybenzoylhydrazone of 2-hydroxybenzoylhydrazine
SCS salicylaldehyde o-hydroxybenzoylhydrazone MFCD00043494 N'-Salicylidenesalicylic hydrazide SALICYLIDENE SALICYLHYDRAZONE salicylaldehyde 2-hydroxybenzoylhydrazone 1-Salicylidene-2-salicyloylhydrazine EINECS 221-773-2 Salicylidene salicylhydrazide 1-Salicyloyl-2-salicylidenehydrazine SALICYLIC (2-HYDROXYBENZYLIDENE)HYDRAZIDE 2-HYDROXYBENZYLIDENE SALICYLHYDRAZIDE 2-hydroxybenzoylhydrazine 2-hydroxybenzoylhydrazone 2-Hydroxybenzylidene 2-hydroxybenzhydrazide |
| Description | SCS (Salicylidene salicylhydrazide) is a potent, allosteric and selective inhibitor of β1-containing GABAA receptors with an IC50 of 32 nM against α2β1γ1θ by VIPR measurement. SCS is also a chelator of metal ions[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 32 nM (α2β1γ1θ; by VIPR measurement) IC50: 4.5 nM (α2β1γ1θ), 5.3 nM (α2β1γ1), 7.9 nM (α1β1γ2s) (Measured by using whole-cell patch clamp)[1] |
| In Vitro | SCS (0.1 nM-3 μM) 对表达 α2β1γ1θ、α2β1γ1 和 α1β1γ2s 受体的 Ltk- 细胞的 GABA EC20 电流有浓度依赖性抑制作用,而对 α2β3γ2s 和 α1β2γ2s 受体无影响[1]。 SCS 的抑制不依赖于电压或使用 (not voltage or use dependent)[1]。 SCS 抑制 GABAA 受体所必需的结构决定因素位于 β1 亚基的精氨酸 238 和甘氨酸 335 区域。β1 亚基的 T255 和 I308 对于 SCS 的抑制是必需的[1]。 |
| In Vivo | SCS (Salicylidene salicylhydrazide; 500-1000 mg/kg, i.p. or 800-1000 mg/kg, oral) 使小鼠产生腹部收缩[2]。 SCS (10-75 mg/kg; i.p.; once) 在小鼠中显示出抗强直性和阶段性疼痛和辣椒素痛觉的抗痛觉活性[2]。 SCS (10-75 mg/kg; i.p.; once) 显示小鼠抗炎活性[2]。SCS (50 and 75 mg/kg; i.p.; once) 显示对神经性痛觉的抗痛觉活性[2]。 Animal Model: BALB/c mice; tonic, phasic and Capsaicin (HY-10448) nociception model[2] Dosage: 10, 25, 50, and 75 mg/kg Administration: IP, single dose Result: Produced a significant protection on tonic, phasic and capsaicin nociception in a dose-dependent manner. Animal Model: BALB/c mice, Oxaliplatin (HY-17371)-induced neuropathic nociception model[2] Dosage: 50 and 75 mg/kg Administration: IP, single dose Result: Significantly attenuated the paw withdrawal threshold changes associated with Oxaliplatin. Significantly increased the percent antinociception during 30-120 min. |
| References |
| Density | 1.406g/cm3 |
|---|---|
| Boiling Point | 427.3ºC at 760mmHg |
| Melting Point | 280-284°C |
| Molecular Formula | C14H12N2O3 |
| Molecular Weight | 256.25700 |
| Flash Point | 212.2ºC |
| Exact Mass | 256.08500 |
| PSA | 81.92000 |
| LogP | 2.25260 |
| Appearance | solid | off-white |
| Index of Refraction | 1.615 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ~20 mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2928000090 |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |

![(6Z)-6-[(5E)-5-(6-oxocyclohexa-2,4-dien-1-ylidene)-1,3,4-oxadiazolidin-2-ylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/308/2491-96-5.png)