72873-74-6
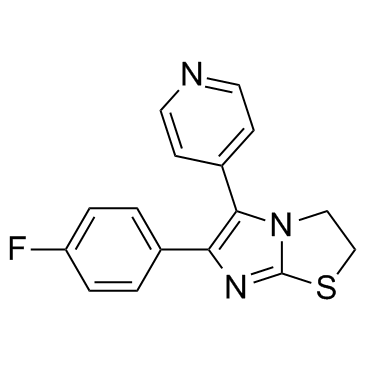
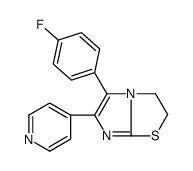
| Name | 6-(4-fluorophenyl)-5-pyridin-4-yl-2,3-dihydroimidazo[2,1-b][1,3]thiazole |
|---|---|
| Synonyms |
5-(4-pyridyl)-6-(4-fluorophenyl)-2,3-dihydroimidazo(2,1-b)-thiazole
MFCD00869367 6-(4-Fluorophenyl)-5-(4-pyridinyl)-2,3-dihydroimidazo[2,1-b][1,3]thiazole 6-(4-Fluorophenyl)-5-(pyridin-4-yl)-2,3-dihydroimidazo[2,1-b][1,3]thiazole SKF-86002 |
| Description | SKF-86002 is a potent inhibitor of p38 MAP kinase wit IC50 of 0.5-1 uM; inhibits LPS-induced IL-1 and TNF-α production in human monocytes (IC50 = 1 μM).IC50 value:Target: p38 MAPK inhibitorin vitro: SKF-86002 inhibited prostaglandin H2 (PGH2) synthase activity (IC50 120 microM) as well as prostanoid production by rat basophilic leukemia (RBL-1) cells (IC50 70 microM) and its sonicate (IC50 100 microM) and human monocytes (IC50 1 microM). In addition, SK&F 86002 inhibited the generation of dihydroxyeicosatetraenoic acid (diHETE) and 5-hydroxyeicosatetraenoic acid (5-HETE) by a high speed supernatant fraction of RBL-1 cells (IC50 10 microM) [1]. differentiation of HL-60 cells toward the neutrophil phenotype resulted in a loss in c-Jun NH2-terminal kinase activation with concomitant acquisition of formylmethionylleucylphenylalanine-stimulatable and stress-inducible p38 MAPK activity as well as apoptosis blockade by SKF-86002 [2]. SKF-86002 blocked superoxide anion production in response to FMLP and reduced adhesion and chemotaxis in response to PAF or FMLP [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 476.1±55.0 °C at 760 mmHg |
| Melting Point | 189-190ºC(lit.) |
| Molecular Formula | C16H12FN3S |
| Molecular Weight | 297.350 |
| Flash Point | 241.7±31.5 °C |
| Exact Mass | 297.073608 |
| PSA | 56.01000 |
| LogP | 1.90 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.713 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934100090 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |




![6-(4-fluorophenyl)-5-pyridin-4-yl-2,3-dihydroimidazo[2,1-b][1,3]thiazole 1-oxide structure](https://image.chemsrc.com/caspic/492/72873-77-9.png)