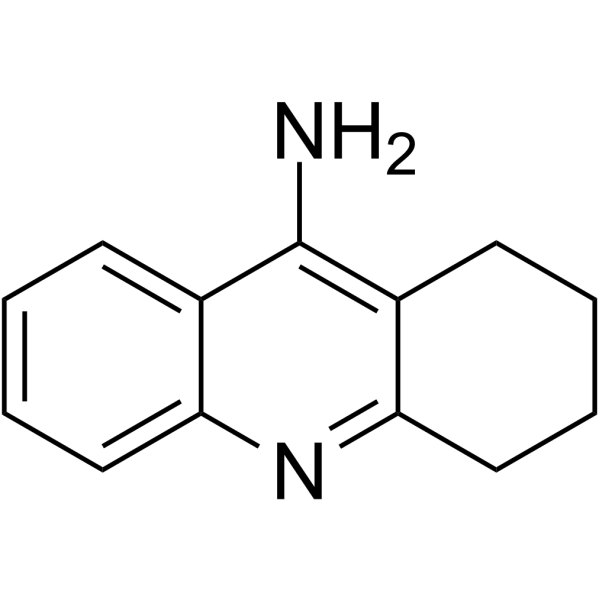
1684-40-8
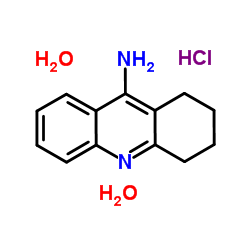
| Name | 9-Amino-1,2,3,4-tetrahydroacridine Hydrochloride Dihydrate |
|---|---|
| Synonyms |
1,2,3,4-Tetrahydroacridin-9-amine hydrochloride (1:1)
1,2,3,4-Tetrahydroacridin-9-aminhydrochlorid 9-Acridinamine, 1,2,3,4-tetrahydro-, hydrochloride, hydrate (1:1:2) 1,2,3,4-tetrahydroacridin-9-amine hydrochloride Cognex 9-Amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate 1,2,3,4-Tetrahydro-9-acridinamine hydrochloride (1:1) THA 1,2,3,4-tetrahydroacridin-9-amine,hydrochloride 1,2,3,4-Tetrahydro-9-acridinamine hydrochloride dihydrate EINECS 216-867-5 MFCD00012657 9-Acridinamine, 1,2,3,4-tetrahydro-, hydrochloride (1:1) 1,2,3,4-tétrahydroacridin-9-amine chlorhydrate Tacrin hydrochloride |
| Description | Tacrine hydrochloride is a potent inhibitor of both AChE and BChE, with IC50s of 31 nM and 25.6 nM, respectively. Tacrine hydrochloride is also a NMDAR inhibitor, with an IC50 of 26 μM. Tacrine hydrochloride can be used for the research of Alzheimer’s disease[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 31 nM (AChE), 25.6 nM (BChE), 26 μM (NMDAR)[1][2] |
| In Vitro | Tacrine (12.5-37.5 nM) inhibits venom acetylcholinesterase as well as human serum butyrylcholinesterase in a concentration-dependent manner[1]. Tacrine reduces the neurotoxicity induced by the activation of the NMDARs in murine cortical neuronal cultures with an IC50of ~500 μM[2]. Tacrine inhibits the NMDAR responses in a concentration-dependent manner with an IC50 of ~190 μM at -60 mV[2]. |
| In Vivo | Tacrine (20-40 μmol/kg; s.c.) disrupts retention of learning in 17- and 30-day old mice in passive avoidance, and while the low dose of tacrine treatment (5 μmol/kg; s.c.) improves retention in 17-day old mice[2]. Tacrine (0.1-0.4 mg/mL; i.p. for 7 d) inhibits the expression of AChE, but does not significantly improve the protection of the retina function and morphology in mice[3]. |
| References |
| Boiling Point | 409.4ºC at 760mmHg |
|---|---|
| Melting Point | 280-284 °C(lit.) |
| Molecular Formula | C13H15ClN2 |
| Molecular Weight | 270.755 |
| Flash Point | 230.5ºC |
| Exact Mass | 270.113495 |
| PSA | 38.91000 |
| LogP | 4.07900 |
| Vapour Pressure | 6.49E-07mmHg at 25°C |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | Missing Phrase - N15.00950417-P305 + P351 + P338 |
| Hazard Codes | T: Toxic;Xn: Harmful; |
| Risk Phrases | 25-36/37/38-20/22 |
| Safety Phrases | S26-S36-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AR9532500 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2933990090 |
|
~% 
1684-40-8 |
| Literature: European Journal of Medicinal Chemistry, , vol. 77, p. 343 - 350 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |