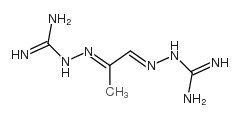
459-86-9
| Name | mitoguazone |
|---|---|
| Synonyms |
Mitoguazone
Me-GAG Pyruvaldehyde bis(amidinohydrazone) 2,2'-(1-Methyl-1,2-ethanediylidene)bis(hydrazinecarbimidamide) methylglyoxal bis-(guanylhydrazone) 1,1'-((methylethanediylidene)dinitrilo)diguanidine 2,2'-(1-methyl-1,2-ethanediylidene)bis-hydrazinecarboximidamid methyl-g MGBG methyl-gag 1,1'-((methylethanediylidene)dinitrilo)di-guanidin |
| Description | Mitoguazone (Methylglyoxal-bis(guanylhydrazone)) is a synthetic polycarbonyl derivative with potent antineoplastic activity. Mitoguazone is a brain-penetrant and competitive S-adenosyl-methionine decarboxylase (SAMDC) inhibitor that disrupts polyamine biosynthesis. Mitoguazone induces cell apoptosis. Mitoguazone inhibits HIV DNA integration into the cellular DNA in both monocytes and macrophages. Mitoguazone has the potential for acute leukemia, Hodgkin's and non-Hodgkin's lymphoma treatment[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Mitoguazone competitively inhibits spermidine synthesis in lymphocytes at concentrations as low as 0.5 μg/mL. Levels of 30 μg/mL or more inhibit protein synthesis and mitochondrial respiration[1]. The ability of Mitoguazone to induce apoptosis by inhibiting the polyamine pathway is assessed in three Burkitt's lymphoma cell lines (Raji, Ramos and Daudi) and one prostate carcinoma cell line (MPC 3). Mitoguazone induces apoptosis in all the different human cancer cell lines tested in a concentration- and time-dependent way, and triggers a p53-independent programmed cell death in the human breast cancer MCF7 cell line[2]. |
| In Vivo | The influence of different stages of leukemia P388 on the pharmacokinetics of the antineoplastic agent Mitoguazone in mice is investigated. Independent of the tumor stage investigated, the total clearance of mitoguazone is slightly reduced reflecting a moderate increase of AUC in the serum of leukemia-bearing animals. Furthermore, in an advanced tumor stage the drug levels in kidneys, liver, spleen and serum are found to be elevated to some extent in comparison to tumor-free controls in contrast to an earlier stage of leukemia[5]. |
| References |
| Density | 1.55g/cm3 |
|---|---|
| Boiling Point | 436.6ºC at 760mmHg |
| Molecular Formula | C5H12N8 |
| Molecular Weight | 184.20200 |
| Flash Point | 217.8ºC |
| Exact Mass | 184.11800 |
| PSA | 148.52000 |
| LogP | 0.69610 |
| Vapour Pressure | 7.99E-08mmHg at 25°C |
| Index of Refraction | 1.692 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|