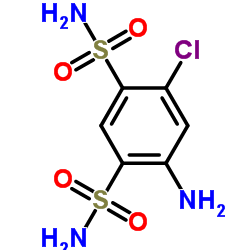
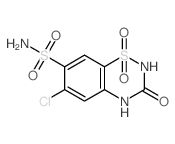
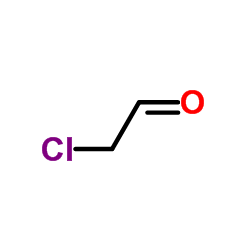
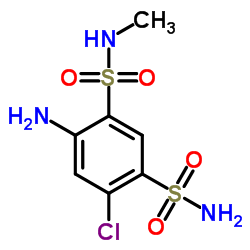
135-07-9
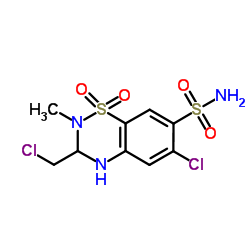
| Name | 6-chloro-3-(chloromethyl)-2-methyl-1,1-dioxo-3,4-dihydro-1λ6,2,4-benzothiadiazine-7-sulfonamide |
|---|---|
| Synonyms |
Methycyclothiazide
6-chloro-3-(chloromethyl)-3,4-dihydro-2-methyl-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-di-oxide 6-Chloro-3-(chloromethyl)-2-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide methyl chlothiazide EINECS 205-172-2 Enduronum Enduron UNII:L3H46UAC61 Aquatensen Duretic methylclothiazide 6-chloro-3-(chlorométhyl)-2-méthyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxyde 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3-(chloromethyl)-3,4-dihydro-2-methyl-, 1,1-dioxide methychlothiazide methyclothiazide Aquaresen Naturon MFCD00242610 Methyclothiazid |
| Description | Methyclothiazide is an orally active antihypertensive agent and a diuretic agent. Methyclothiazide leads to a reduction of the vascular response to the action of endogenous vasoconstricting stimuli, such as Norepinephrine (HY-13715). Methyclothiazide is against voltage-dependent Ca-channel (VDCC) activity in vitro[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: voltage-dependent Ca-channel (VDCC) |
| In Vitro | Methyclothiazide (0-100 μM) induces endothelium-dependent inhibition of the vasoconstrictor responses to NE and AVP only in aortas from SHR, and the maximal vasoconstrictive effect of Norepinephrine (HY-13715) and arginine vasopressin (AVP) is decreased by 59% and 32.3 %, respectively[1]. Methyclothiazide (0-100 μM) induces inhibitory effect on the contractile response to Norepinephrine (HY-13715) is abolished by N-nitro-L-arginine (NOLA) but not indomethacin[1]. Methyclothiazide (100 μM) affects the vascular responses to extracellular Ca2+ under high-K+ depolarizing conditions. It can reduce Ca2+ contracture in a high-K+, Ca2+-free solution. The maximal contracture is reduced by 90.4%[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 597.9±60.0 °C at 760 mmHg |
| Melting Point | 223-225ºC |
| Molecular Formula | C9H11Cl2N3O4S2 |
| Molecular Weight | 360.237 |
| Flash Point | 315.4±32.9 °C |
| Exact Mass | 358.956787 |
| PSA | 126.33000 |
| LogP | 1.76 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.606 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
135-07-9 |
| Literature: Journal of the American Chemical Society, , vol. 82, p. 1132 - 1135 |
|
~% 
135-07-9 |
| Literature: Journal of the American Chemical Society, , vol. 82, p. 1132 - 1135 |
|
~% 
135-07-9 |
| Literature: Journal of the American Chemical Society, , vol. 82, p. 1132 - 1135 |
|
~% 
135-07-9 |
| Literature: Journal of the American Chemical Society, , vol. 82, p. 1132 - 1135 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |