41607-20-9
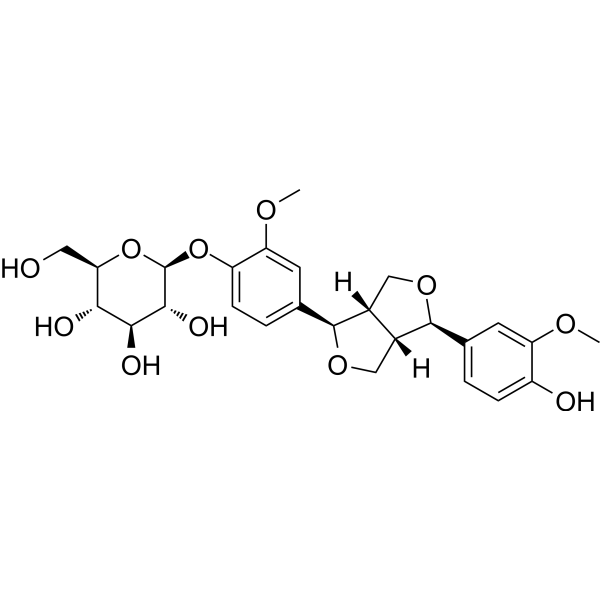
| Name | (2S,3R,4S,5S,6R)-2-(4-((1R,3aS,4R,6aS)-4-(4-hydroxy-3-methoxyphenyl)tetrahydro-1H,3H-furo[3,4-c]furan-1-yl)-2-methoxyphenoxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol |
|---|---|
| Synonyms |
4-[(1R,3aS,4R,6aS)-4-(4-Hydroxy-3-methoxyphenyl)tetrahydro-1H,3H-furo[3,4-c]furan-1-yl]-2-methoxyphenyl β-D-glucopyranoside
β-D-Glucopyranoside, 2-methoxy-4-[(1R,3aS,4R,6aS)-tetrahydro-4-(4-hydroxy-3-methoxyphenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenyl |
| Description | (-)-Pinoresinol 4-O-glucoside ((-)-Pinoresinol 4-O-β-D-glucopyranoside) is a potent and orally active α-glucosidase inhibitor with an IC50 value of 48.13 µM. (-)-Pinoresinol 4-O-glucoside increases cell migration and early differentiation of pre-osteoblasts. (-)-Pinoresinol 4-O-glucoside increases protein level of BMP2, p-Smad1/5/8, RUNX2. (-)-Pinoresinol 4-O-glucoside attenuates oxidative stress, hyperglycemia and hepatic toxicity. (-)-Pinoresinol 4-O-glucoside has the potential for the research of osteoporosis and periodontal disease[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 48.13 µM (α-Glucosidase)[1] |
| In Vitro | (-)-Pinoresinol 4-O-glucoside (0、10、30 µM;24 小时) 在含有 50 μg/mL 的成骨补充培养基 (OS) 中增加前成骨细胞分化过程中的细胞迁移[1]< /sup>. (-)-Pinoresinol 4-O-glucoside (10、30 µM;7 天) 在前成骨细胞分化过程中增加早期分化并增加矿化结节的形成[1]。 (-)-Pinoresinol 4-O-glucoside (10、30 µM;3 天) 增加前成骨细胞中 BMP2、ALP、OCN mRNA 水平的表达[1]。 (-)-Pinoresinol 4-O-glucoside (10、30 µM;3 天) 增加 BMP2、p-Smad1/5/8、RUNX2 的蛋白表达水平[1]。 RT-PCR[1] Cell Line: pre-osteoblasts Concentration: 10, 30 µM Incubation Time: 3 days Result: Upregulated the mRNA level of BMP2 and its target osteoblast genes, ALP and osteocalcin (OCN). Western Blot Analysis[1] Cell Line: pre-osteoblasts Concentration: 10, 30 µM Incubation Time: 3 days Result: Enhanced protein level of BMP2, followed by the phosphorylation of Smad1/5/8 and the expression of RUNX2. |
| In Vivo | (-)-Pinoresinol 4-O-glucoside (50 mg/kg;口服;20 天) 减轻小鼠的氧化应激、高血糖和肝毒性[2]。 Animal Model: 27-30 g, Male Swiss albino mice[2] Dosage: 50 mg/kg Administration: P.o.; twenty days Result: Exhibited a hepatoprotective activity in vivo as it lowered AST and ALT levels, caused a prominent decline in serum glucose level by 37.83% in streptozotocin-treated mice with promising elevation in insulin level of 25.37%. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 752.5±60.0 °C at 760 mmHg |
| Molecular Formula | C26H32O11 |
| Molecular Weight | 520.53 |
| Flash Point | 408.9±32.9 °C |
| Exact Mass | 520.194458 |
| PSA | 156.53000 |
| LogP | -0.69 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Hazard Codes | Xi |
|---|