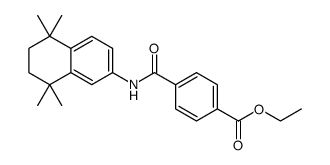
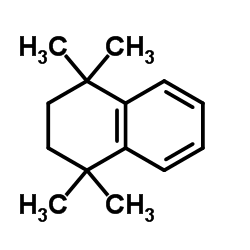
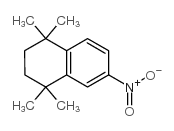
94497-51-5
| Name | tamibarotene |
|---|---|
| Synonyms |
4-[(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-2-naphthalenyl)carbamoyl]benzoic acid
4-[(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl]benzoic acid 4-((5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl)benzoic acid 4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalen-2-yl)carbamoyl]benzoic acid tamibarotene |
| Description | Tamibarotene is a retinoic acid receptor α/β (RARα/β) agonist, showing high selectivity over RARγ. |
|---|---|
| Related Catalog | |
| Target |
RARα/β[1] |
| In Vitro | Tamibarotene (20, 40 μM) down-regulates expression of cell cycle gene in T-cell lymphoma cells. Tamibarotene (5 μM) increases RARE activity in RARA-overexpressing cells to a much greater degree than in RARAlow cells. Moreover, RARAwt overexpression augments the degree of CDK2, CDK4, and CDK6 inhibition caused by Tamibarotene treatment[1]. Tamibarotene directly reverses the profibrotic phenotype of transforming growth factor-β1-treated dermal fibroblasts, suppresses ICAM-1 expression in endothelial cells, and promots M1 macrophage differentiation in vitro[2]. Tamibarotene (4 μM) up-regulates apelin mRNA and protein levels dose-dependently in VSMCs. Upon Tamibarotene stimulation, the RARα (retinoic acid receptor α) is recruited to the apelin promoter by interacting with KLF5 and Sp1 prebound to the TCE site of the apelin promoter to form a transcriptional activation complex, subsequently leading to the up-regulation of apelin expression in VSMCs. KLF5 and Sp1 co-operatively mediate Tamibarotene-induced apelin expression through their direct binding to the TCE on the apelin promoter[4]. |
| In Vivo | Tamibarotene (1 mg/kg/day) significantly attenuates dermal and hypodermal fibrosis in bleomycin (BLM)-treated mice and tight skin 1 mice, respectively. Consistently, Tamibarotene significantly suppresses the expression of various molecules related to tissue fibrosis, including transforming growth factor-β1, connective tissue growth factor, IL-4, IL-10, IL-13, IL-17A, tumor necrosis factor-α, IFN-γ, and monocyte chemotactic protein 1 in the lesional skin of BLM-treated mice. Furthermore, Tamibarotene decreases the proportion of effector T cells, while increasing that of naive T cells among CD4+ T cells in the draining lymph nodes of BLM-treated mice[2]. Tamibarotene (2.5 mg/kg, p.o.) does not result in any significant alteration of the AST, ALT, or ALP serum levels in periodontitis-challenged mice compared with that in untreated mice. Tamibarotene ameliorates alveolar bone resorption, significantly reduces the number of P. gingivalis-induced osteoclasts in mice. Tamibarotene measurably increases the percentage of CD4+ Foxp3+ Treg cells as compared to those in EPD mice. Tamibarotene is also effective in reducing the expression of CD4+ROR-γt+ (Th17) cells in P. gingivalis-infected gingival tissues and CLNs[3]. Tamibarotene (1 mg/kg, p.o.) increases apelin expression in balloon-injured arteries of rats, consistent with the results from the cultured VSMCs[4]. In aged SAMP8 mice, hippocampal ADAM10 levels improve after Tamibarotene (1 mg/kg/day) administration. Hes5 and Ki67 are restored and spatial working memory also improves after Tamibarotene administration[5]. |
| Cell Assay | The CellTiter Aqueous Non-Radioactive Cell Proliferation Assay Kit is used to assess cell growth. Briefly, 10,000 cells per well are seeded in a 96-well plate and cultured in RPMI containing 2% charcoal-stripped FBS and indicated retinoid concentrations for 72 hours. At the end of the treatment period, the MTS reagent is added, cells are incubated an additional 2-4 hours, and absorbance is measured at 490 nanometers. |
| Animal Admin | For the infection, mice are given sulfamethoxazole and trimethoprim in an oral suspension at 10 mL of deionized water ad libitum for 10 days to reduce the native flora and to support colonization of P. gingivalis W83. Four days after the antibiotic therapy finishes, periodontal infection is established through oral inoculation using 1010 colony-forming units of P. gingivalis suspended in 100 μL 4% carboxymethyl cellulose (CMC) for 7 days. The mice are euthanized 4 weeks after the first oral inoculation. Tamibarotene (2.5 mg/kg) is suspended in a 0.5% carboxymethyl cellulose solution. The drug is orally gavaged into the esophagus daily in a volume of 0.1 mL/10 g body weight. Tamibarotene is administered 1 h before the induction of periodontitis and then given daily per the protocol until day 28. Control mice with periodontal disease receive the same volume of 0.5% carboxymethyl cellulose solution. The body weight of each mouse is measured every 3 days. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 449.6±45.0 °C at 760 mmHg |
| Melting Point | 231-232ºC |
| Molecular Formula | C22H25NO3 |
| Molecular Weight | 351.439 |
| Flash Point | 225.7±28.7 °C |
| Exact Mass | 351.183441 |
| PSA | 66.40000 |
| LogP | 6.48 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.593 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Tamibarotene Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Tamibarotene CAS number:94497-51-5 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Store in closed vessels. Storage: Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data No data Melting point: Flash point:No data Density:No data Molecular formula:C22H25NO3 Molecular weight:351.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2924299090 |
|
~89% 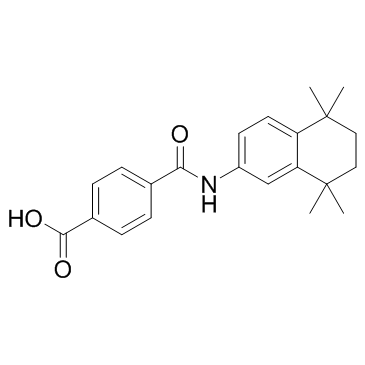
94497-51-5 |
| Literature: Fang, Weiwei; Deng, Qinyue; Xu, Mizhi; Tu, Tao Organic Letters, 2013 , vol. 15, # 14 p. 3678 - 3681 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
|
~% 
94497-51-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 11 p. 2182 - 2192 |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |