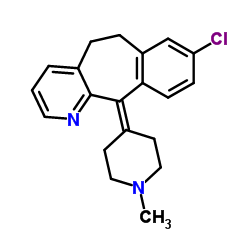
117796-52-8
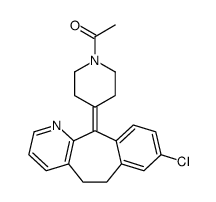
| Name | 1-[4-(8-chloro-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridin-11-ylidene)piperidin-1-yl]ethanone |
|---|---|
| Synonyms |
N-Acetyldesloratadine
PegCNTF RESPATADINE Loratadine Impurity 11 |
| Description | N-Acetyldesloratadine (SCH-37370) is a potent, orally active dual antagonist of platelet-activating factor (PAF) and histamine. N-Acetyldesloratadine inhibits PAF-induced aggregation of human platelets, with an IC50 of 0.6 µM[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.257g/cm3 |
|---|---|
| Boiling Point | 546.9ºC at 760mmHg |
| Molecular Formula | C21H21ClN2O |
| Molecular Weight | 352.85700 |
| Flash Point | 284.5ºC |
| Exact Mass | 352.13400 |
| PSA | 33.20000 |
| LogP | 4.21570 |
| Vapour Pressure | 5.15E-12mmHg at 25°C |
| Index of Refraction | 1.626 |
| Storage condition | -20°C |
| Precursor 10 | |
|---|---|
| DownStream 0 | |