198470-84-7
| Name | parecoxib |
|---|---|
| Synonyms |
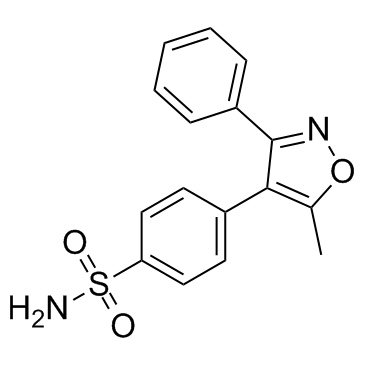
N-{[4-(5-Methyl-3-phenyl-1,2-oxazol-4-yl)phenyl]sulfonyl}propanamide
N-[[4-[5-methyl-3-phenylisoxazol-4-yl]phenyl]sulfonyl]propanamide parecoxib N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]-propanamide 4-[4-(N-propionylsulfamoyl)-phenyl]-5-methyl-3-phenyl-isoxazole N-propanoyl-4-(5-methyl-3-phenylisoxazol-4-yl)benzenesulfonamide N-[[4-[5-methyl-3-phenylisoxazol-4-yl]phenyl]sulfonyl]acetamide Dynastat |
| Description | Parecoxib is a potent and selective COX-2 inhibitor.IC50 value:Target: COX-2in vitro: The prodrug Parecoxib as well as its active metabolite val have a specific affinity to the cannabinoid (CB) receptor measured in CB1-expressing HEK 293 cells and rat brain tissue [1].in vivo: Adult male Sprague-Dawley rats were administered parecoxib (10 or 30 mg kg(-1), IP) or isotonic saline twice a day starting 24 h after middle cerebral artery occlusion (MCAO) for three consecutive days [2]. The selective COX-2 inhibitor parecoxib was delivered 20 min before or 20 min after the incision by intraperitoneal injection. Pretreatment with parecoxib markedly attenuated the pain hypersensitivity induced by incision [3]. |
|---|---|
| Related Catalog | |
| Target |
COX-2 |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 148.9-151° |
| Molecular Formula | C19H18N2O4S |
| Molecular Weight | 370.422 |
| Exact Mass | 370.098724 |
| PSA | 101.14000 |
| LogP | 1.72 |
| Index of Refraction | 1.580 |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361d-H373 |
| Precautionary Statements | P281 |
| Hazard Codes | Xn,N |
| Risk Phrases | 63-48/22-51/53 |
| Safety Phrases | 36/37-61 |
| RIDADR | NONH for all modes of transport |
| RTECS | TX1478700 |
|
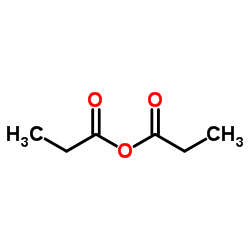
~82% 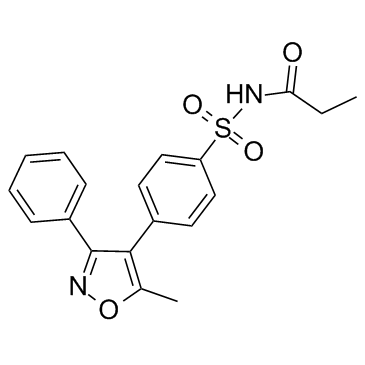
198470-84-7 |
| Literature: Talley, John J.; Bertenshaw, Stephen R.; Brown, David L.; Carter, Jeffery S.; Graneto, Matthew J.; Kellogg, Michael S.; Koboldt, Carol M.; Yuan, Jinhua; Zhang, Yan Y.; Seibert, Karen Journal of Medicinal Chemistry, 2000 , vol. 43, # 9 p. 1661 - 1663 |
|
~75% 
198470-84-7 |
| Literature: Letendre, Leo J.; Kunda, Sastry A.; Gallagher, Donald J.; Seaney, Lisa M.; McLaughlin, Kathleen Patent: US2003/105334 A1, 2003 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |