75225-51-3
| Name | mevinolinic acid |
|---|---|
| Synonyms |
2-methoxy-4,4'-dihydroxychalcone
Echinantin 4',4-dihydroxy-2-methoxychalcone LOVASTATIN HYDROXY ACID, SODIUM SALT Echinatin Mevinolinic acid lovastatin acid loureirin C lovastatin hydroxy acid |
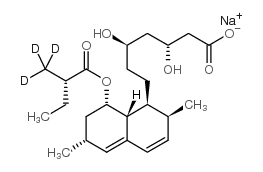
| Description | Lovastatin acid (Mevinolinic acid), an active metabolite of Lovastatin, is a potent competitive HMG-CoA reductase inhibitor with a Ki of 0.6 nM[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.6 nM (HMG-CoA reductase)[1] |
| References |
| Density | 1.14g/cm3 |
|---|---|
| Boiling Point | 602.3ºC at 760 mmHg |
| Molecular Formula | C24H38O6 |
| Molecular Weight | 422.55 |
| Flash Point | 199.1ºC |
| Exact Mass | 447.26800 |
| PSA | 106.89000 |
| LogP | 2.38090 |
| Index of Refraction | 1.537 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 4 | |
|---|---|
| DownStream 1 | |