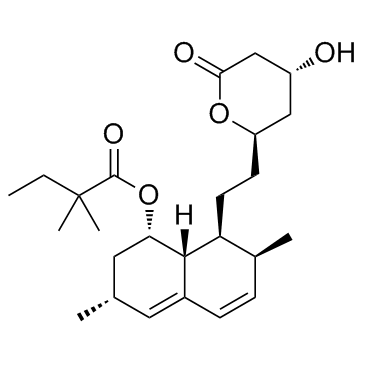
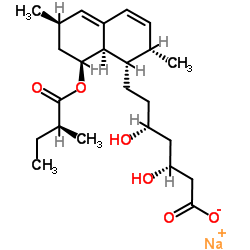
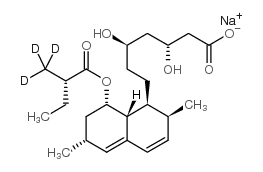
75330-75-5
| Name | lovastatin |
|---|---|
| Synonyms |
MEVINOLIN
LOVALIP (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl-(2S)-2-methylbutanoat Lovastatin 1,2,6,7,8,8a-Hexahydro-b,d-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoic Acid d-Lactone 2b,6a-Dimethyl-8a-(2-methyl-1-oxobutoxy)mevinic Acid Lactone (+)-Mevinolin (2S)-2-Methylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester LOVASTIN Altocor MEVACOR Antibiotic MB 530B Altoprev Rovacor 6a-Methylcompactin Sivlor (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl (S)-2-Methylbutyrate (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl (2S)-2-methylbutanoate MFCD00072164 mevlor [1S-[1a(R*),3a,7b,8b(2S*,4S*),8ab]]-2-Methylbutanoic Acid1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl Ester msd803 Paschol Simvastatin Impurity 5 |
| Description | Lovastatin is a cell-permeable HMG-CoA reductase inhibitor used to lower cholesterol. |
|---|---|
| Related Catalog | |
| Target |
HMG-CoA reductase[1] |
| In Vitro | Lovastatin is an inactive lactone prodrug that must be chemically or enzymatically converted to its dihydroxy open-acid form in order to elicit inhibitory activity. Lovastatin in its hydroxy acid form is an exceptionally potent competitive inhibitor of liver HMG CoA reductase[1]. Lovastatin, other than its anticholesterol property, has diverse applications in the field of osteoporosis, neuro-degeneration, rheumatoid arthritis, antifungals and also is reported to reduce proliferation of lung cancer cells, breast cancer (MCF-7), liver cancer (HepG2). Lovastatin treatments show significant dose dependent cytotoxic effect on HeLa cells with IC50 value of 160 μg/mL. Lovastatin is effective to accelerate hydroxyl radical scavenging activity (54.06%) at an IC50 of 3601 μg/mL[2]. |
| In Vivo | Lovastatin is an inactive lactone that is hydrolyzed in the liver to an active f3-hydroxyacid form. This principal metabolite is the inhibitor of the enzyme HMG-CoA reductase. The Ki is 1 nM. Lovastatin and its β-hydroxyacid metabolite are highly bound to human plasma proteins. Lovastatin crosses the blood-brain and placental barriers[3]. Lovastatin produces a profound reduction of apolipoprotein-B-containing lipoproteins, especially LDL cholesterol and, to a lesser extent, plasma triglycerides, and a small increase in HDL cholesterol[4]. |
| Cell Assay | Hela cells are treated with lovastatin (0, 5, 10, 20, 40, 80, 160, 320 μg/mL) for 24 h. Cells treated with culture medium serves as a negative control. cell viability is measured using the MTT based colorimetric assay [2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 559.2±50.0 °C at 760 mmHg |
| Melting Point | 175°C |
| Molecular Formula | C24H36O5 |
| Molecular Weight | 404.540 |
| Flash Point | 185.3±23.6 °C |
| Exact Mass | 404.256287 |
| PSA | 72.83000 |
| LogP | 4.07 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.532 |
| Storage condition | 2-8°C |
| Water Solubility | 0.0004 mg/mL at 25 ºC |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 3077 |
| WGK Germany | 3 |
| RTECS | EK7907000 |
| Packaging Group | III |
| Hazard Class | 9 |
| HS Code | 2932999099 |
| Precursor 2 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



![6(R)-[2-[1,2,6,7,8,8a(R)-Hexahydro-2(S),6(R)-dimethyl-8(S)-[[2(S)-methylbutyryl]oxy]-1(S)-naphtyl]ethyl]-3,4,5,6-tetrahydro-4(R)-[(tert-butyldimethylsilyl)oxy]-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/013/79691-11-5.png)
![4-(TERT-BUTYLDIMETHYLSILANYLOXY)-6-[2-(8-HYDROXY-2,6-DIMETHYL-1,2,6,7,8,8A-HEXAHYDRONAPHTHALEN-1-YL)ETHYL]TETRAHYDROPYRAN-2-ONE structure](https://image.chemsrc.com/caspic/414/79902-31-1.png)