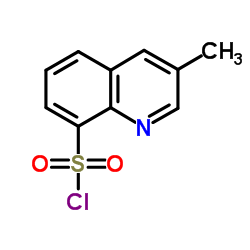
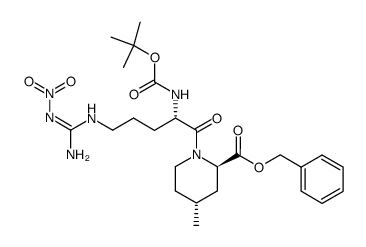
74863-84-6
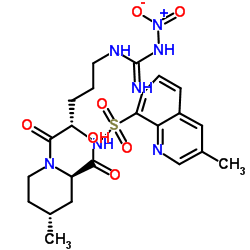
| Name | Argatroban anhydrous |
|---|---|
| Synonyms |
Novastan
(2R,4R)-4-Methyl-1-{N-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]arginyl}-2-piperidinecarboxylic acid Argatroban (2R,4R)-4-Methyl-1-{N-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonyl]arginyl}piperidine-2-carboxylic acid (2R,4R)-1-{N-(Diaminomethylene)-N-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-L-ornithyl}-4-methyl-2-piperidinecarboxylic acid 2-Piperidinecarboxylic acid, 1-[(2S)-5-[(aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-, (2R,4R)-, hydrate (1:1) (2R,4R)-1-{N-(diaminomethylidene)-N-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonyl]-L-ornithyl}-4-methylpiperidine-2-carboxylic acid (2R,4R)-4-Methyl-1-{N-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonyl]-L-arginyl}piperidine-2-carboxylic acid (2R,4R)-1-(5-Carbamimidamido-2-{[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]amino}pentanoyl)-4-methyl-2-piperidinecarboxylic acid MQPA Argipidine (2R,4R)-4-Methyl-1-[N2-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl]-2-piperidinecarboxylic Acid Slonnon [2R-[1(2S*),2a,4b]]-[partial]-1-[5-[(Aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2-piperidinecarboxylic Acid (2R,4R)-4-Methyl-1-{N-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-L-arginyl}-2-piperidinecarboxylic acid hydrate (1:1) Argatroban monohydrate MFCD00866789 Argatroban anhydrous UNII:IY90U61Z3S UNII:OCY3U280Y3 (2R,4R)-4-Methyl-1-[(S)-N2-[[(R,S)-1,2,3,4-tetrahydro-3-methyl-8-quinolinyl]sulfonyl]arginyl]pipecolic Acid |
| Description | Argatroban is a direct, selective thrombin inhibitor.Target: ThrombinArgatroban may have a complementary effect for preventing thrombus formation without aggravating bleeding tendency because of its monotarget specificity to thrombin. Administration (0.5 to 2 micrograms/kg/min) of argatroban is a safe anticoagulant for left heart bypass in repairs of traumatic aortic rupture associated with multiple organ injuries [1]. Argatroban, as compared with heparin, appears to enhance reperfusion with TPA in patients with AMI, particularly in those patients with delayed presentation. The incidences of major bleeding and adverse clinical outcome were lower in the patients receiving argatroban [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 801.3±75.0 °C at 760 mmHg |
| Melting Point | 188-1890C |
| Molecular Formula | C23H36N6O5S |
| Molecular Weight | 508.634 |
| Flash Point | 438.4±37.1 °C |
| Exact Mass | 508.246796 |
| PSA | 186.09000 |
| LogP | 2.56 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Storage condition | Store at +4°C |
| Hazard Codes | Xn,N |
|---|---|
| Risk Phrases | R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . |
| Safety Phrases | S28-S61-S28A |
| RIDADR | UN 2020 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | SK2450000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2935009090 |
|
~95% 
74863-84-6 |
| Literature: LUNDBECK PHARMACEUTICALS ITALY S.P.A. Patent: WO2009/124906 A2, 2009 ; Location in patent: Page/Page column 20-21 ; |
|
~90% 
74863-84-6 |
| Literature: Cossy, Janine; Belotti, Damien Bioorganic and Medicinal Chemistry Letters, 2001 , vol. 11, # 15 p. 1989 - 1992 |
|
~% 
74863-84-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 15 p. 1989 - 1992 |
|
~% 
74863-84-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 15 p. 1989 - 1992 |
|
~% 
74863-84-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 15 p. 1989 - 1992 |
|
~% 
74863-84-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 15 p. 1989 - 1992 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |