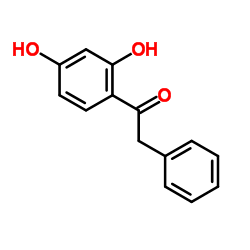
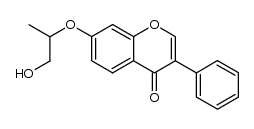
35212-22-7
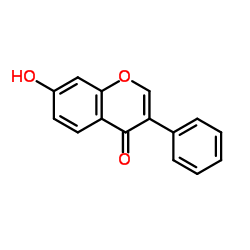
| Name | ipriflavone |
|---|---|
| Synonyms |
MFCD00221719
Iprosten 3-phenyl-7-(propan-2-yloxy)-4H-chromen-4-one Osteofix 7-Isopropoxyisoflavone 3-phenyl-7-propan-2-yloxychromen-4-one 4-isopropoxyisoflavone 7-Isopropoxy-3-phenyl-4H-1-benzopyran-4-one Ipriflavone (JAN) 7-Isopropoxy-3-phenyl-4H-chromen-4-one Ipriflavone 7-isopropoxy-isoflavone 7-isopropyloxy-isoflavone Osten Yambolap Ipriflavone (Osteofix) FL-113 Osteoquine EINECS 201-641-0 7-Isopropoxy-3-phenylchromone |
| Description | Ipriflavone is a synthetic isoflavone derivative used to suppress bone resorption. |
|---|---|
| Related Catalog | |
| In Vitro | Ipriflavone inhibits the proliferation and DNA synthesis of MDA-231 cells and blocks the ligand-induced phosphorylation of Tyr(845) of the EGFR. Ipriflavone does not promote apoptosis of MDA-231 cells[1]. Ipriflavone also promotes the deposition of calcium and the formation of mineralized nodules by newborn rat calvarial osteoblast-like cells as well as the activity of alkaline phosphatase[2]. |
| In Vivo | Daily oral administration of ipriflavone at 12 mg/mouse significantly inhibits the development of new osteolytic bone metastases and the progression of established osteolytic lesions, prolonging the life of tumor-bearing mice. Ipriflavone reduces the number of osteoclasts at the bone-cancer interface with no severe adverse effects on the host[1]. 1-month treatment with ipriflavone increases bone density and improves the biomechanical properties of adult rat male bones without altering mineral composition[3] |
| Cell Assay | Ipriflavone is dissolved in absolute ethanol and added to the medium at 1-50 μM. The final ethanol concentrations are <0.5% (v/v). MDA-231 cells are seeded in 35 mm culture dishes in DMEM supplemented with 10% FBS in the presence of ipriflavone (1, 10 or 50 μM) or ethanol. After 48 hr incubation, the medium is changed with fresh medium containing the same concentrations of ipriflavone or ethanol. After incubation for 24, 48, 72 and 96 hr from the initial seeding, cells are treated with trypan blue to estimate the number of viable cells[1]. |
| Animal Admin | Rats: To assess the potential impact of ipriflavone on the biomechanical properties and mineral composition of bone, adult male rats are orally administered two doses (200 or 400 mg/kg bw) of ipriflavone for 1 month. Bone biomechanics are evaluated by vibration damping, an index of strain energy loss, and impact strength[3]. Mice: Ipriflavone is suspended in water containing 0.5% (w/v) methylcellulose and given to mice orally at 6 or 12 mg/0.2 mL daily, which is equivalent to 200 or 400 mg/kg body weight. MDA-231 cells are injected s.c. into the interscapular space of nude mice (on day 0), which are then given ipriflavone (6 or 12 mg/mouse) or the methylcellulose solution orally from day 1 to day 27. Tumor growth is analyzed twice a week by measuring the tumor volume[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 435.9±45.0 °C at 760 mmHg |
| Melting Point | 116-120 °C(lit.) |
| Molecular Formula | C18H16O3 |
| Molecular Weight | 280.318 |
| Flash Point | 209.3±15.1 °C |
| Exact Mass | 280.109955 |
| PSA | 39.44000 |
| LogP | 4.37 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.592 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DJ3100500 |
| HS Code | 2932999099 |
|
~94% 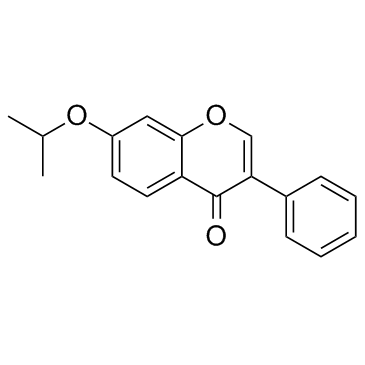
35212-22-7 |
| Literature: Oetvoes, Ferenc; Toth, Geza; Szatmari, Istvan; Levai, Ferenc Journal of Labelled Compounds and Radiopharmaceuticals, 1999 , vol. 42, # 5 p. 497 - 504 |
|
~96% 
35212-22-7 |
| Literature: Erregierre S.p.A. Patent: US5973169 A1, 1999 ; |
|
~96% 
35212-22-7 |
| Literature: Vasil'ev, S.A.; Golubushina, G.M.; Kabachnyi, V.I.; Luk'yanchikov, M.S.; Molchanov, G.I.; et al. Pharmaceutical Chemistry Journal, 1990 , vol. 24, # 9 p. 641 - 645 Khimiko-Farmatsevticheskii Zhurnal, 1990 , vol. 24, # 9 p. 38 - 41 |
|
~93% 
35212-22-7 |
| Literature: Muthukrishnan; Singh, Om V. Synthetic Communications, 2008 , vol. 38, # 22 p. 3875 - 3883 |
|
~% 
35212-22-7 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 11, # 18 p. 4069 - 4081 |
| Precursor 6 | |
|---|---|
| DownStream 5 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |