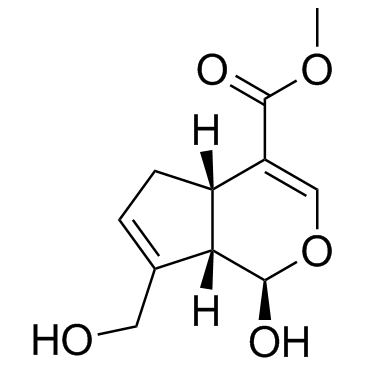
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
GY5828000
-
CHEMICAL NAME :
-
Cyclopenta(c)pyran-4-carboxylic acid, 1,4a-alpha,5,7a-alpha-tetrahydro-1-hydroxy-7- (hydroxymethyl)-, methyl ester
-
CAS REGISTRY NUMBER :
-
6902-77-8
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C11-H14-O5
-
MOLECULAR WEIGHT :
-
226.25
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>50 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 19,259,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
237 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 94,157,1974
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
190 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 94,157,1974
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
153 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. (Nippon Yakugakkai, 2-12-15 Shibuya, Shibuya-ku, Tokyo 150, Japan) No.1- 1881- Volume(issue)/page/year: 94,157,1974
|