Genipin
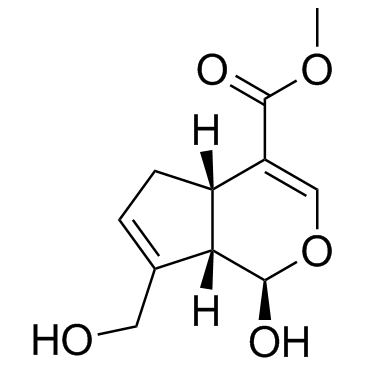
Genipin structure
|
Common Name | Genipin | ||
|---|---|---|---|---|
| CAS Number | 6902-77-8 | Molecular Weight | 226.226 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 416.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H14O5 | Melting Point | 106-108 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 164.9±22.2 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of GenipinGenipin is a natural water soluble crosslinking reagent. |
| Name | Genipin |
|---|---|
| Synonym | More Synonyms |
| Description | Genipin is a natural water soluble crosslinking reagent. |
|---|---|
| Related Catalog | |
| In Vitro | Genipin stimulats glucose uptake in a time- and dose-dependent manner. The maximal effect is achieved at 2 h with a concentration of 10 μM. In myotubes, genipin promotes glucose transporter 4 (GLUT4) translocation to the cell surface, which increases the phosphorylation of insulin receptor substrate-1 (IRS-1), AKT, and GSK3β. Meanwhile, genipin increases ATP levels, closed KATP channels, and then increases the concentration of calcium in the cytoplasm in C2C12 myotubes. Genipin-stimulated glucose uptake could be blocked by both the PI3-K inhibitor wortmannin and calcium chelator EGTA. Moreover, genipin increases the level of reactive oxygen species and ATP in C2C12 myotubes[1]. Genipin increases mitochondrial membrane potential, which then increases ATP levels and closes KATP channels, thereby stimulating insulin secretion in pancreatic β-cells. Genipin activates glucose-excited POMC neurons[2]. Cytochrome c content increases significantly in the cytosol of genipin-treated FaO cells. Activation of caspase-3 and caspase-7 is ultimately responsible for genipin-induced apoptotic process in hepatoma cells. ROS level notably increases in Hep3B cells treated with 200 μM genipin[3]. |
| Kinase Assay | Briefly, the peptide substrate N-acetyl-Asp-Glu-Val-Asp-ρ-nitroanilide (Ac-DEVD-ρNA) is added to the cell lysates in assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM dithiothreitol, 1 mM EDTA, 10% glycerol) and incubated at 37°C. The cleavage of the substrate is monitored at 405 nm. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 416.0±45.0 °C at 760 mmHg |
| Melting Point | 106-108 °C(lit.) |
| Molecular Formula | C11H14O5 |
| Molecular Weight | 226.226 |
| Flash Point | 164.9±22.2 °C |
| Exact Mass | 226.084122 |
| PSA | 75.99000 |
| LogP | 0.12 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.565 |
| InChIKey | AZKVWQKMDGGDSV-BCMRRPTOSA-N |
| SMILES | COC(=O)C1=COC(O)C2C(CO)=CCC12 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H319 |
| Precautionary Statements | P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| Safety Phrases | 22 |
| RIDADR | UN 2811 6.1/PG 3 |
| RTECS | GY5828000 |
| HS Code | 2942000000 |
| HS Code | 2942000000 |
|---|
|
Dielectric behavior of gelatine-glycosaminoglycans blends: an impedance analysis.
Mater. Sci. Eng. C. Mater. Biol. Appl. 33(4) , 2455-9, (2013) The dielectric behavior of the gelatine-GAGs based blend systems has been studied to understand the dynamic behavior of the water at the protein-GAGs interfaces which are relevant for tissue engineeri... |
|
|
Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs.
Biomaterials 52 , 452-62, (2015) There is a critical need to engineer a neotrachea because currently there are no long-term treatments for tracheal stenoses affecting large portions of the airway. In this work, a modular tracheal tis... |
|
|
Genipin crosslinked ethyl cellulose-chitosan complex microspheres for anti-tuberculosis delivery.
Colloids Surf. B Biointerfaces 103 , 530-7, (2013) Genipin-crosslinked complex microspheres made of the combination of two polymers, ethyl cellulose and chitosan, were prepared by spray drying method. Rifabutin, an anti-tuberculosis agent was used as ... |
| Methyl (1R,4aS,7aS)-1-hydroxy-7-(hydroxymethyl)-1,4a,5,7a-tetrahydrocyclopenta[c]pyran-4-carboxylate |
| MFCD00888600 |
| Cyclopenta[c]pyran-4-carboxylic acid, 1,4a,5,7a-tetrahydro-1-hydroxy-7-(hydroxymethyl)-, methyl ester, (1R,4aS,7aS)- |
| EINECS 300-006-4 |

