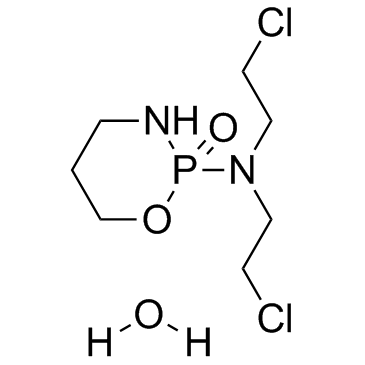
6055-19-2
| Name | cyclophosphamide hydrate |
|---|---|
| Synonyms |
CCRIS 7469 {Hydrate}
MFCD00149395 Cytoxan N,N-bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide hydrate 2H-1,3,2-Oxazaphosphorine, 2-[bis(2-chloroethyl)amino]tetrahydro-, 2-oxide, monohydrate Cyclophosphamide Monohydrate EINECS 200-015-4 UNII-8N3DW7272P 2H-1,3,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-, 2-oxide, hydrate (1:1) CYCLOPHOSPHAMIDE HYDRATE N,N-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide hydrate (1:1) Endoxon N,N-bis(2-chloroethyl)-2-oxo-1,3,2λ<sup>5</sup>-oxazaphosphinan-2-amine,hydrate Cyclophosphamide (hydrate) |
| Description | Cyclophosphamide hydrate is a synthetic alkylating agent chemically related to the nitrogen mustards with antineoplastic and immunosuppressive activities. |
|---|---|
| Related Catalog | |
| In Vitro | Cyclophosphamide induces outer membrane blebbing, leads to DNA fragmentation, as revealed by TUNEL staining of free 3'-OH DNA ends, and induces cleavage of the caspase 3 and caspase 7 substrate PARP in 9L/P450 cells. Bcl-2 expression fully blocks the activation of both initiator caspases as well as the effector caspase 3 in cells treated with activated Cyclophosphamide. Bcl-2 inhibits the cytotoxic effects but not the cytostatic effects of activated Cyclophosphamide[1]. Cyclophosphamide inhibits the AChE reversibly with an IC50 of 511 μM[2]. Carbon tetrachloride does not affect the direct cytotoxicity of cyclophosphamide or 4-hydroxycyclophosphamide to cells in culture[3]. |
| Kinase Assay | 9L cells are treated with drug for the times indicated in each experiment. Floating and attached cells are collected, pooled, resuspended in lysis buffer (10 mM HEPES buffer, pH 7.4, containing 2 mM EDTA, 0.1% CHAPS detergent, 5 mM DTT, 350 ng/mL phenylmethylsulfonyl fluoride, 10 ng/mL pepstatin A, 10 ng/mL aprotinin, and 20 ng/mL leupeptin) and lysed by three freeze-thaw cycles (alternating between a dry ice isopropanol bath and a 37°C water bath). Lysates are spun in a bench top centrifuge at full speed for 15 min and the supernatant (cell extract) fraction transferred to a new tube. Cell extracts (20 μL) are assayed for caspase 9, caspase 8, and caspase 3 activity by incubation at 37°C for either 1 h (caspase 3) or 3 h (caspase 9 and caspase 8) in 500 μL of reaction buffer (10 mM HEPES, pH 7.4, 2 mM EDTA, 0.1% CHAPS, and 5 mM DTT) containing 50 μM caspase form-selective substrate: Ac-LETD-AFC for caspase 8; Ac-LEHD-AFC for caspase 9; and Ac-DEVD-AMC for caspase 3. Background activity is determined for each sample as follows. Cell extracts are preincubated for 15 min at room temperature, with or without caspase form-selective inhibitor: 1 μM z-LETD-FMK for caspase 8, 1 μM z-LEHD-FMK for caspase 9, and 5 μL of Casputin for caspase 3. Caspase activity measured in the absence of inhibitor is divided by the background caspase activity measured in the presence of inhibitor. A value of 1 is subtracted from each measured activity, such that a caspase activity of 0 corresponds to no increase in the specific caspase activity with drug treatment. Fluorescence of the caspase product (excitation at 395 nm and emission at 525 nm for AFC substrates, and excitation at 380 nm and emission at 460 nm for the AMC substrate) is measured using a Shimadzu model RF-1501 spectrofluorophotometer and the manufacturer's PC-1501 software package. |
| Cell Assay | 9L/pBabe, 9L/Bax, and 9L/Bcl-2 cells are treated with 12, 24, or 50 μM MFA for 72 h. Cells remaining on the plates at 0, 24, 48, and 72 h are washed twice with cold PBS and then stained for 5 min with crystal violet [1.25 g of crystal violet dissolved in a solution containing 50 mL of 37% formaldehyde and 450 mL of methanol]. The stained cells are washed three times in tap water and the plates are allowed to dry. The stain is eluted from the cells with 70% ethanol and the absorbance is then read at 595 nm. The staining intensity of each drug-treated sample (A 595) is then graphed as a percentage of the staining intensity at the 0-h time point. |
| References |
| Boiling Point | 336.1ºC at 760 mmHg |
|---|---|
| Melting Point | 49-51 °C(lit.) |
| Molecular Formula | C7H17Cl2N2O3P |
| Molecular Weight | 279.101 |
| Flash Point | >230 °F |
| Exact Mass | 278.035370 |
| PSA | 60.61000 |
| LogP | 2.14850 |
| Storage condition | 2-8°C |
| Water Solubility | 40 g/L |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H350 |
| Precautionary Statements | P201-P301 + P310 + P330-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25;R36/37/38;R45;R46;R61 |
| Safety Phrases | S53-S45-S37/39-S26 |
| RIDADR | UN 3464 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | RP6157750 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 2924299090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |