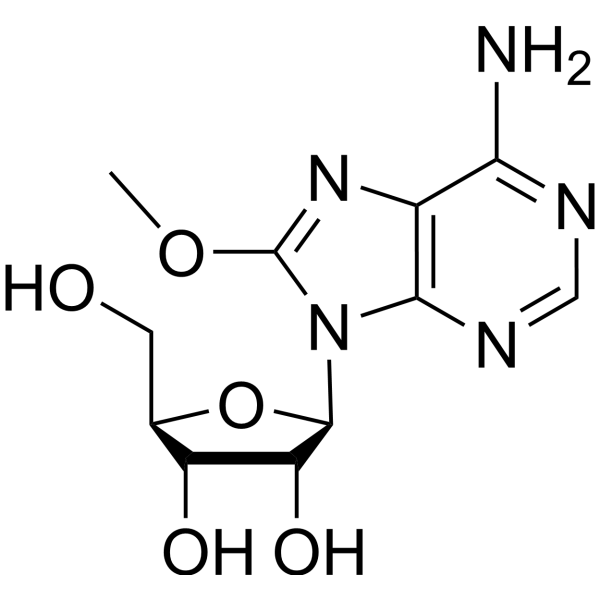
3868-33-5
| Name | 8-aminoadenosine |
|---|---|
| Synonyms |
8-Amino-adenosin
8-Amino Adenosine 8-Aminoadenosine 8-Amino-D-adenosine |
| Description | 8-Aminoadenosine (8-NH2-Ado), a RNA-directed nucleoside analogue, reduces cellular ATP levels and inhibits mRNA synthesis. 8-Aminoadenosine blocks Akt/mTOR signaling and induces autophagy and apoptosis in a p53-independent manner. 8-Aminoadenosine has antitumor activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Akt mTOR |
| In Vitro | 8-Aminoadenosine (8-NH2-Ado; 0.1-10 μM; for 48 h) has IC50s of 1.5 μM and 8.88 μM in MM.1S and U266 cells, respectively[1]. 8-Aminoadenosine (10 μM; for 24 h) induces significant apoptotic death of MCF-7 cells in p53-independent pathway. 8-Aminoadenosine causes PARP cleavage in MCF-7 cells[2]. 8-Aminoadenosine (3 μM; 0.5-4 h) induces autophagy in the MM.1S cell line[1]. 8-Aminoadenosine (3 μM; 2-16 h) causes a greater drop in ATP levels in the MM.1S cells[1]. 8-Aminoadenosine (3 μM; 5 h) causes a 50% reduction in glucose consumption in MM.1S cells[1]. 8-Aminoadenosine (3 μM; 5 h) indicates a time-dependent decrease in GLUT1 expression at 5 h, whereas at 24 h there was a down-regulation of both transporters (GLUT1 and GLUT4) in MM.1S cells[1]. 8-Aminoadenosine inhibits cell proliferation, activated cell death, and does not activate transcription of the p53 target gene p21 or increase protein levels of either p53 or p21[1]. The toxic effects of 8-Aminoadenosine require adenosine kinase activity to convert 8-Aminoadenosine to 8-NH2-ATP in adenosine kinase-deficient cells[1]. Cell Viability Assay[1] Cell Line: MM.1S and U266 cells Concentration: 0.1, 0.3, 1, 3, 10 μM Incubation Time: For 48 hours Result: Had IC50s of 1.5 μM and 8.88 μM in MM.1S and U266 cells, respectively. Apoptosis Analysis[2] Cell Line: MCF-7 cells Concentration: 10 μM Incubation Time: For 24 hours Result: Induced significant apoptotic death. Apoptosis was not inhibited by knockdown of functional p53. Apoptosis Analysis[1] Cell Line: MM.1S cell line Concentration: 3 μM Incubation Time: 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 hours Result: Induced the formation of LC3-II protein. Caused the appearance of a population with a high AVO content with 1 μM for 24 hours. |
| References |
| Density | 2.25g/cm3 |
|---|---|
| Boiling Point | 747.1ºC at 760mmHg |
| Melting Point | 180-185ºC dec. |
| Molecular Formula | C10H14N6O4 |
| Molecular Weight | 282.26 |
| Flash Point | 405.6ºC |
| Exact Mass | 282.10800 |
| PSA | 165.56000 |
| Appearance | white to beige |
| Storage condition | -20?C Freezer |
| Water Solubility | H2O: soluble2mg/mL, clear (warmed) |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T |
| Risk Phrases | 25 |
| Safety Phrases | 45 |
| RIDADR | UN 2811 6.1 / PGIII |
| Precursor 5 | |
|---|---|
| DownStream 0 | |