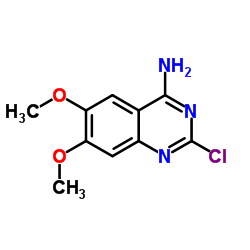
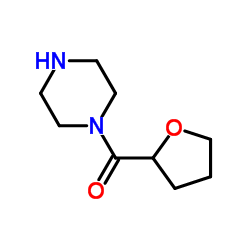
63590-64-7
| Name | terazosin |
|---|---|
| Synonyms |
Terazosin Hydrochloride
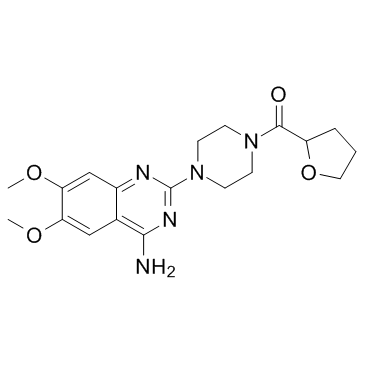
[4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone MFCD00467965 |
| Description | Terazosin is a selective alpha1-antagonist used for treatment of symptoms of benign prostatic hyperplasia (BPH).Target: Alpha-1 Adrenergic ReceptorTerazosin is selective for alpha 1A-adrenoceptors which appear to dominate in the human prostate; the therapeutic relevance of this selectivity remains to be assessed in clinical studies [1]. Administration of terazosin 1 mg orally for 28 d. Terazosin initially shifted the dose-response curve of phenylephrine to the right, with a significant increase in ED50 for phenylephrine from a control value of 102 to 759 ng/min on day 1 of terazosin (P < 0.001). The mean Kd of terazosin was estimated as 11 +/- 15 nM in the first few days of treatment. This study demonstrates that pharmacological tolerance to the alpha 1-adrenoceptor blocking action of terazosin occurs in man and may be responsible for loss in efficacy with chronic therapy [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.332 g/cm3 |
|---|---|
| Boiling Point | 664.5ºC at 760 mmHg |
| Melting Point | 281-283°C |
| Molecular Formula | C19H25N5O4 |
| Molecular Weight | 387.43300 |
| Flash Point | 355.7ºC |
| Exact Mass | 387.19100 |
| PSA | 103.04000 |
| LogP | 1.64090 |
| Storage condition | -20°C Freezer |
| Water Solubility | H2O: 25 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TK8044925 |
|
~% 
63590-64-7 |
| Literature: US2007/161791 A1, ; Page/Page column 2; 3-4 ; |
|
~% 
63590-64-7 |
| Literature: US6313293 B1, ; |
|
~% 
63590-64-7 |
| Literature: Synthetic Communications, , vol. 34, # 10 p. 1881 - 1884 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |